Snapshot of Protein Structure Evolution Reveals Conservation of Functional Dimerization through Intertwined Folding
Chirgadze, D.Y., Demydchuk, M., Becker, M., Moran, S., Paoli, M.(2004) Structure 12: 1489-1494
- PubMed: 15296742
- DOI: https://doi.org/10.1016/j.str.2004.06.011
- Primary Citation of Related Structures:
1U36, 1U3J, 1U3Y, 1U3Z, 1U41, 1U42 - PubMed Abstract:
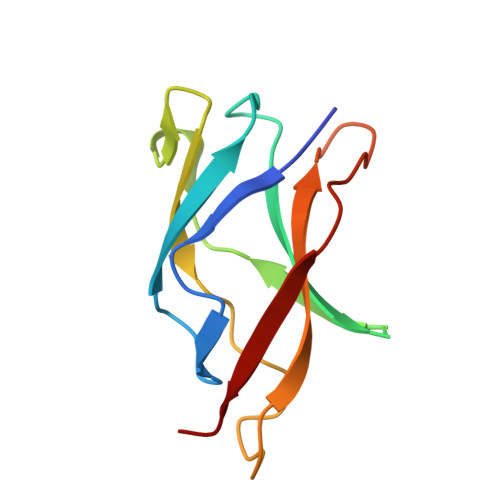
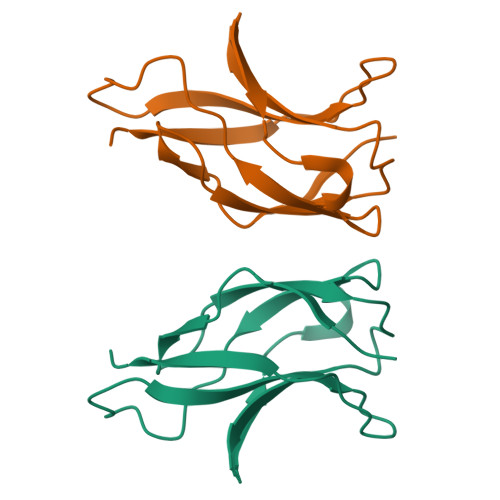
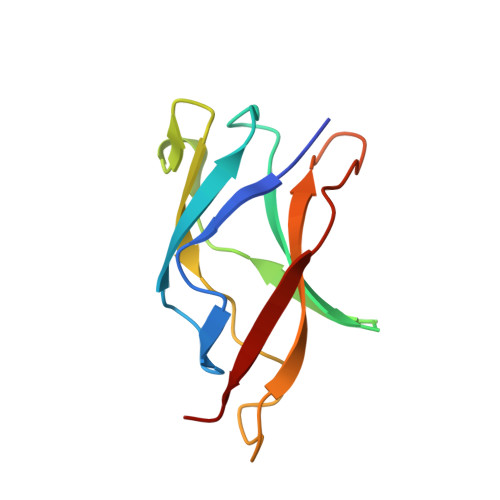
Protein-protein interactions govern a wide range of cellular processes. Molecular recognition responsible for homodimerization and heterodimerization in the rel/NF-kappaB family of eukaryotic transcription factors relies on a small cluster of hydrophobic residues. We have carried out a structural analysis of six NF-kappaB p50 dimer interface mutants; one of them revealed a remarkable alteration. One or possibly both its mutations cause a switch into an intertwined dimer, in which the molecular partners exchange nearly half of their fold. In spite of the extensive swapping of secondary structure elements, the topology within each counterpart is preserved, with a very similar overall structure and minimal changes at the interface. Thus intertwining rescues structure and function from a destabilizing mutation. Since the mutants originate from a directed evolution experiment and are functional, the data provide an evolutionary snapshot of how a protein structure can respond to mutations while maintaining a functional molecular architecture.
Organizational Affiliation:
Department of Biochemistry, University of Cambridge, Tennis Court Road, Cambridge, CB2 1GA, United Kingdom.