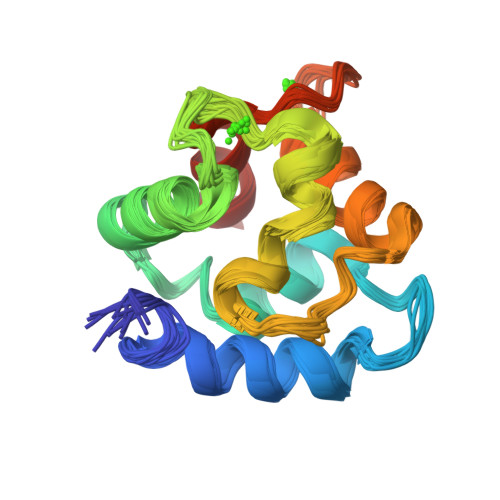
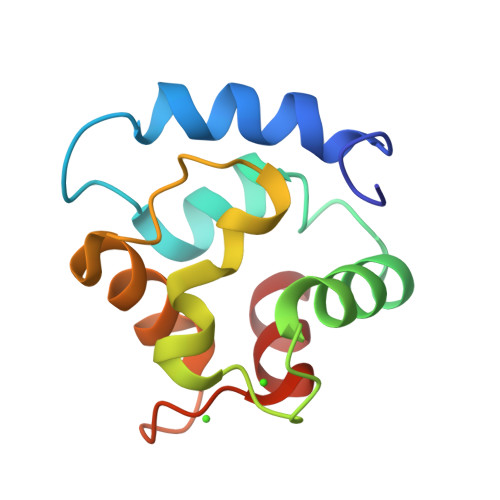
Solution Structure of Human beta-Parvalbumin and Structural Comparison with Its Paralog alpha-Parvalbumin and with Their Rat Orthologs(,)
Babini, E., Bertini, I., Capozzi, F., Del Bianco, C., Hollender, D., Kiss, T., Luchinat, C., Quattrone, A.(2004) Biochemistry 43: 16076-16085
- PubMed: 15610002
- DOI: https://doi.org/10.1021/bi048388o
- Primary Citation of Related Structures:
1TTX - PubMed Abstract:
The aim of this research was to determine the structure of human beta-parvalbumin (109 amino acids) and to compare it with its paralog and ortholog proteins. The structure was determined in solution using multinuclear and multidimensional NMR methods and refined using substitution of the EF-hand Ca(2+) ion with a paramagnetic lanthanide. The resulting family of structures had a backbone rmsd of 0.50 A. Comparison with rat oncomodulin (X-ray, 1.3 A resolution) as well as with human (NMR, backbone rmsd of 0.49 A) and rat (X-ray, 2.0 A resolution) parvalbumins reveals small but reliable local differences, often but not always related to amino acid variability. The analysis of these structures has led us to propose an explanation for the different affinity for Ca(2+) between alpha- and beta-parvalbumins and between parvalbumins and calmodulins.
Organizational Affiliation:
CERM, Via Luigi Sacconi 6, 50019 Sesto Fiorentino, Italy.