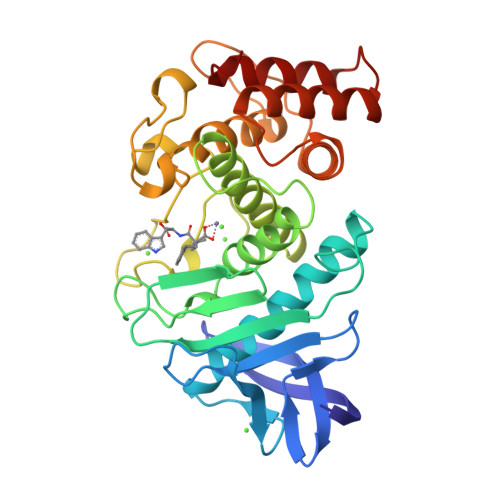
Binding of N-carboxymethyl dipeptide inhibitors to thermolysin determined by X-ray crystallography: a novel class of transition-state analogues for zinc peptidases
Monzingo, A.F., Matthews, B.W.(1984) Biochemistry 23: 5724-5729
- PubMed: 6395881
- DOI: https://doi.org/10.1021/bi00319a010
- Primary Citation of Related Structures:
1TMN - PubMed Abstract:
The mode of binding of the specific thermolysin inhibitor N-(1-carboxy-3-phenylpropyl)-L-leucyl-L-tryptophan (KI approximately 5 X 10(-8) M) [Maycock, A. L., DeSousa, D. M., Payne, L. G., ten Broeke, J., Wu, M. T., & Patchett, A. A. (1981) Biochem. Biophys. Res. Commun. 102, 963-969] has been determined by X-ray crystallography and refined to an R value of 17.1% at 1.9-A resolution. The inhibitor binds to thermolysin with both oxygens of the N-carboxymethyl group liganded to the zinc to give overall pentacoordination of the metal. The bidentate ligation of the inhibitor differs from the monodentate binding seen previously for carboxylate-zinc interactions in thermolysin and is closer to the bidentate geometry observed for the binding of hydroxamates [Holmes, M. A., & Matthews, B. W. (1981) Biochemistry 20, 6912-6920]. The geometry of the inhibitor and its interactions with the protein have a number of elements in common with the presumed transition state formed during peptide hydrolysis. The observed zinc ligation supports the previous suggestion that a pentacoordinate intermediate participates in the mechanism of catalysis. However, the alpha-amino nitrogen of the inhibitor is close to Glu-143, suggesting that this residue might accept a proton from an attacking water molecule (as proposed before) and subsequently donate this proton to the leaving nitrogen. By analogy with thermolysin, it is proposed that a related mechanism should be considered for peptide cleavage by carboxypeptidase A.(ABSTRACT TRUNCATED AT 250 WORDS)