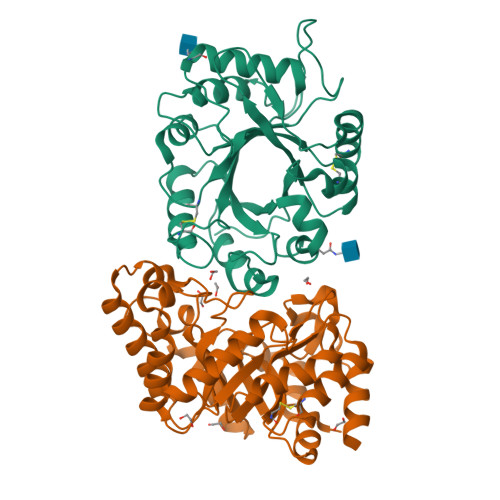
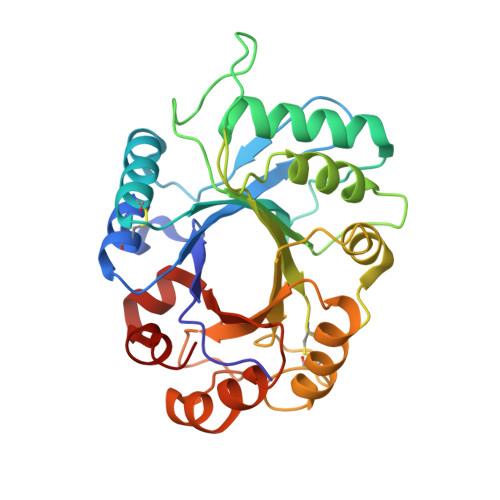
The Dual Nature of the Wheat Xylanase Protein Inhibitor XIP-I: STRUCTURAL BASIS FOR THE INHIBITION OF FAMILY 10 AND FAMILY 11 XYLANASES.
Payan, F., Leone, P., Porciero, S., Furniss, C., Tahir, T., Williamson, G., Durand, A., Manzanares, P., Gilbert, H.J., Juge, N., Roussel, A.(2004) J Biological Chem 279: 36029-36037
- PubMed: 15181003
- DOI: https://doi.org/10.1074/jbc.M404225200
- Primary Citation of Related Structures:
1TA3, 1TE1 - PubMed Abstract:
The xylanase inhibitor protein I (XIP-I) from wheat Triticum aestivum is the prototype of a novel class of cereal protein inhibitors that inhibit fungal xylanases belonging to glycoside hydrolase families 10 (GH10) and 11 (GH11). The crystal structures of XIP-I in complex with Aspergillus nidulans (GH10) and Penicillium funiculosum (GH11) xylanases have been solved at 1.7 and 2.5 A resolution, respectively. The inhibition strategy is novel because XIP-I possesses two independent enzyme-binding sites, allowing binding to two glycoside hydrolases that display a different fold. Inhibition of the GH11 xylanase is mediated by the insertion of an XIP-I Pi-shaped loop (Lalpha(4)beta(5)) into the enzyme active site, whereas residues in the helix alpha7 of XIP-I, pointing into the four central active site subsites, are mainly responsible for the reversible inactivation of GH10 xylanases. The XIP-I strategy for inhibition of xylanases involves substrate-mimetic contacts and interactions occluding the active site. The structural determinants of XIP-I specificity demonstrate that the inhibitor is able to interact with GH10 and GH11 xylanases of both fungal and bacterial origin. The biological role of the xylanase inhibitors is discussed in light of the present structural data.
Organizational Affiliation:
Architecture et Fonction de Macromolécules Biologiques, UMR-6098, CNRS et Universités d'Aix-Marseille I et II, 31 Chemin Joseph Aiguier, 13402 Marseille Cedex 20, France.