Structure of palmitoylated BET3: insights into TRAPP complex assembly and membrane localization
Turnbull, A.P., Prinz, B., Holz, C., Schultchen, J., Lang, C.(2005) EMBO J 24: 875-884
- PubMed: 15692564
- DOI: https://doi.org/10.1038/sj.emboj.7600565
- Primary Citation of Related Structures:
1SZ7 - PubMed Abstract:
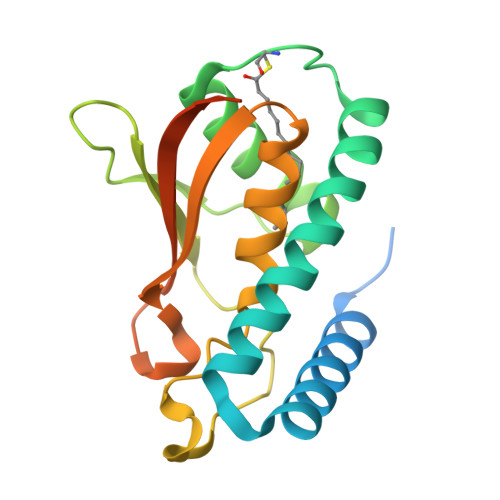
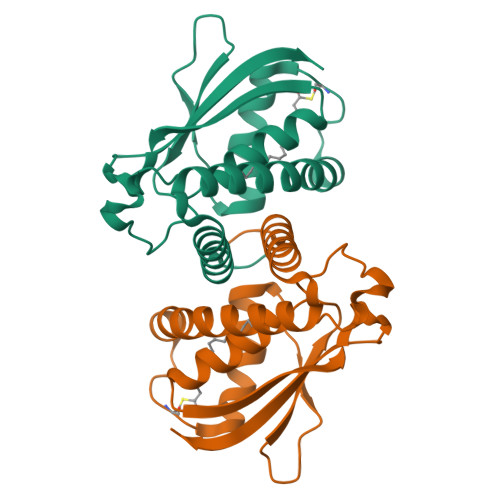
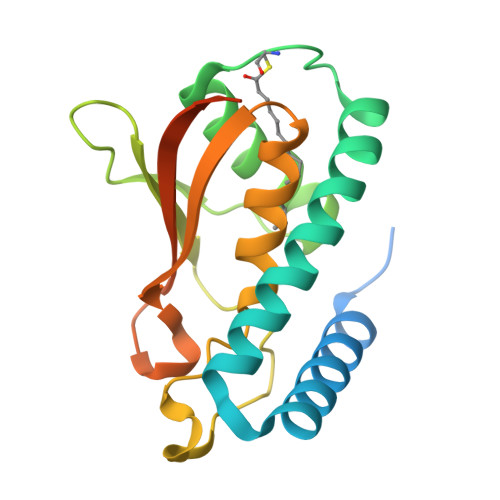
BET3 is a component of TRAPP, a complex involved in the tethering of transport vesicles to the cis-Golgi membrane. The crystal structure of human BET3 has been determined to 1.55-A resolution. BET3 adopts an alpha/beta-plait fold and forms dimers in the crystal and in solution, which predetermines the architecture of TRAPP where subunits are present in equimolar stoichiometry. A hydrophobic pocket within BET3 buries a palmitate bound through a thioester linkage to cysteine 68. BET3 and yeast Bet3p are palmitoylated in recombinant yeast cells, the mutant proteins BET3 C68S and Bet3p C80S remain unmodified. Both BET3 and BET3 C68S are found in membrane and cytosolic fractions of these cells; in membrane extractions, they behave like tightly membrane-associated proteins. In a deletion strain, both Bet3p and Bet3p C80S rescue cell viability. Thus, palmitoylation is neither required for viability nor sufficient for membrane association of BET3, which may depend on protein-protein contacts within TRAPP or additional, yet unidentified modifications of BET3. A conformational change may facilitate palmitoyl extrusion from BET3 and allow the fatty acid chain to engage in intermolecular hydrophobic interactions.
Organizational Affiliation:
Max-Delbrück-Centrum für Molekulare Medizin, Berlin, Germany.