Role of binding site loops in controlling nitric oxide release: structure and kinetics of mutant forms of nitrophorin 4
Maes, E.M., Weichsel, A., Andersen, J.F., Shepley, D., Montfort, W.R.(2004) Biochemistry 43: 6679-6690
- PubMed: 15157102
- DOI: https://doi.org/10.1021/bi049748a
- Primary Citation of Related Structures:
1SXU, 1SXW, 1SXX, 1SXY, 1SY0, 1SY1, 1SY2, 1SY3 - PubMed Abstract:
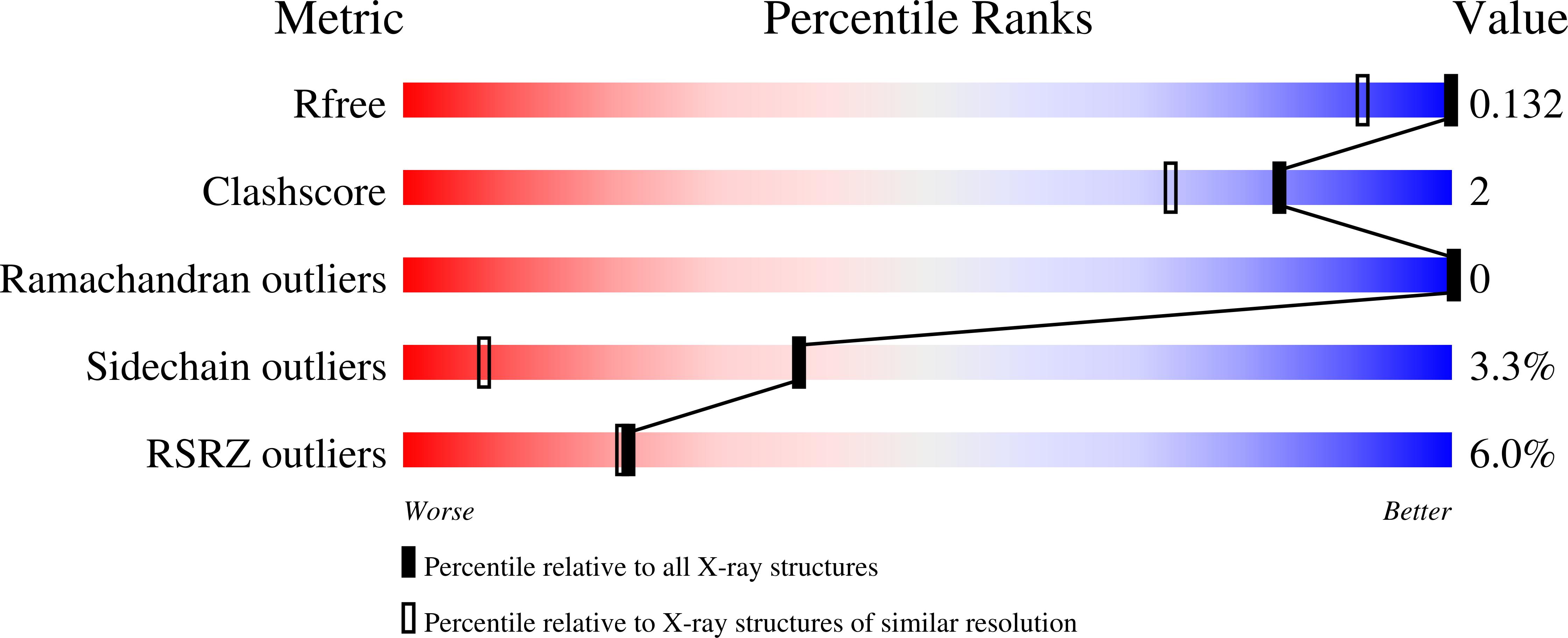
Nitrophorins are ferric heme proteins that transport nitric oxide (NO) from blood-sucking insects to victims. NO binding is tighter at lower pH values, as found in the insect salivary gland, and weaker at the pH of the victim's tissue, facilitating NO release and subsequent vasodilation. Previous structural analyses of nitrophorin 4 (NP4) from Rhodnius prolixus revealed a substantial NO-induced conformational change involving the A-B and G-H loops, which rearrange to desolvate the distal pocket and pack nonpolar residues against the heme-ligated NO. Previous kinetic analyses revealed a slow, biphasic, and pH-dependent NO release, which was proposed to be associated with loop movements. In this study, we created NP4 mutants D30A and D30N (A-B loop), D129A/L130A (G-H loop), and T121V (distal pocket). Eight crystal structures were determined, including complexes with NO, NH(3), and imidazole, to resolutions as high as 1.0 A. The NO-induced conformational change is largely abolished in the loop mutants, but retained in T121V. Kinetic analyses using stopped-flow spectroscopy revealed the pH dependence for NO release is eliminated for D129A/L130A, considerably reduced for D30A and D30N, but retained for T121V. NO association rates were increased 2-5-fold for T121V, but were unchanged in the loop mutants. Taken together, our findings demonstrate that the pH dependency for NO release is linked to loop dynamics and that solvent reorganization is apparently rate-limiting for formation of the initial iron-nitrosyl bond. Interestingly, the multiphasic kinetic behavior of rNPs was not affected by mutations, and its cause remains unclear.
Organizational Affiliation:
Department of Biochemistry and Molecular Biophysics, University of Arizona, Tucson, Arizona 85721, USA.