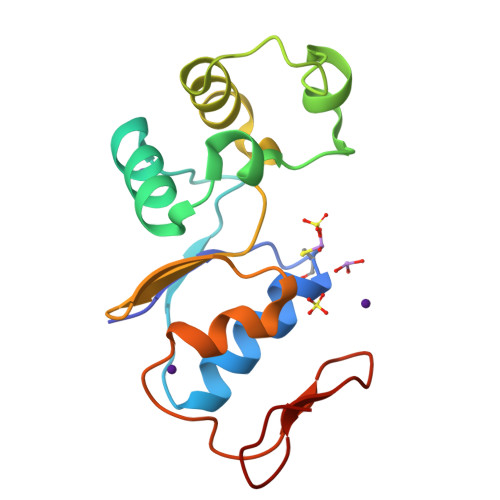
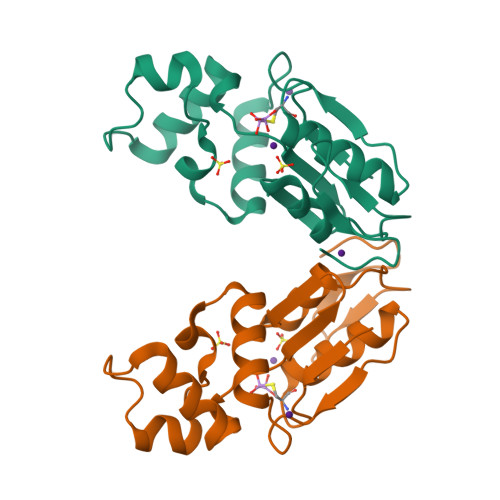
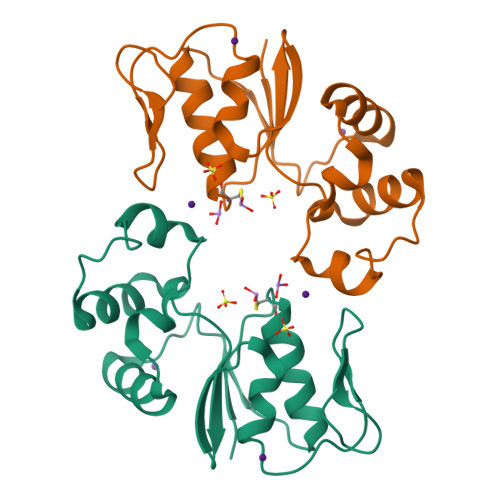
Arginine 60 in the ArsC arsenate reductase of E. coli plasmid R773 determines the chemical nature of the bound As(III) product.
DeMel, S., Shi, J., Martin, P., Rosen, B.P., Edwards, B.F.(2004) Protein Sci 13: 2330-2340
- PubMed: 15295115
- DOI: https://doi.org/10.1110/ps.04787204
- Primary Citation of Related Structures:
1S3C, 1S3D, 1SD8, 1SD9, 1SJZ, 1SK0, 1SK1, 1SK2 - PubMed Abstract:
Arsenic is a ubiquitous environmental toxic metal. Consequently, organisms detoxify arsenate by reduction to arsenite, which is then excreted or sequestered. The ArsC arsenate reductase from Escherichia coli plasmid R773, the best characterized arsenic-modifying enzyme, has a catalytic cysteine, Cys 12, in the active site, surrounded by an arginine triad composed of Arg 60, Arg 94, and Arg 107. During the reaction cycle, the native enzyme forms a unique monohydroxyl Cys 12-thiol-arsenite adduct that contains a positive charge on the arsenic. We hypothesized previously that this unstable intermediate allows for rapid dissociation of the product arsenite. In this study, the role of Arg 60 in product formation was evaluated by mutagenesis. A total of eight new structures of ArsC were determined at resolutions between 1.3 A and 1.8 A, with R(free) values between 0.18 and 0.25. The crystal structures of R60K and R60A ArsC equilibrated with the product arsenite revealed a covalently bound Cys 12-thiol-dihydroxyarsenite without a charge on the arsenic atom. We propose that this intermediate is more stable than the monohydroxyarsenite intermediate of the native enzyme, resulting in slow release of product and, consequently, loss of activity.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Wayne State University School of Medicine, 540 E. Canfield Avenue, Detroit, MI 48201, USA.