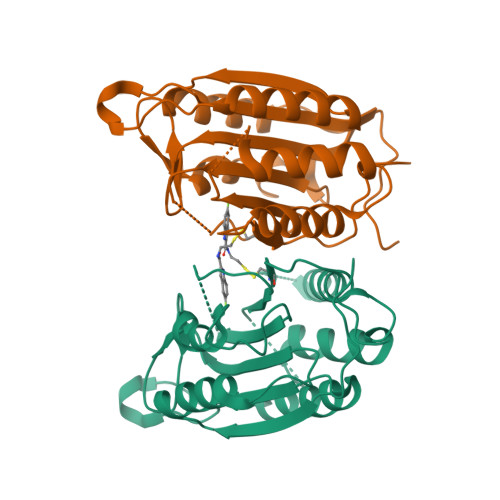
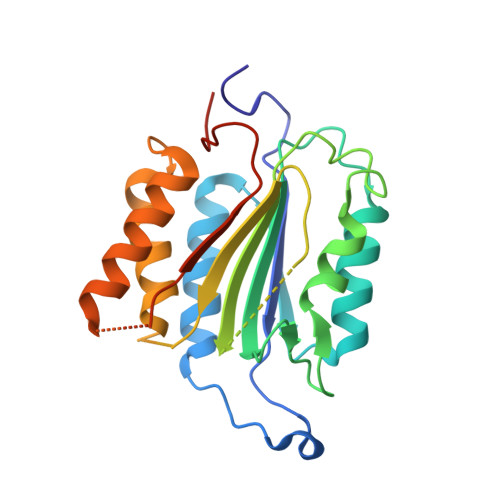
Discovery of an allosteric site in the caspases
Hardy, J.A., Lam, J., Nguyen, J.T., O'Brien, T., Wells, J.A.(2004) Proc Natl Acad Sci U S A 101: 12461-12466
- PubMed: 15314233
- DOI: https://doi.org/10.1073/pnas.0404781101
- Primary Citation of Related Structures:
1SHJ, 1SHL - PubMed Abstract:
Allosteric regulation of proteins by conformational change is a primary means of biological control. Traditionally it has been difficult to identify and characterize novel allosteric sites and ligands that freeze these conformational states. We present a site-directed approach using Tethering for trapping inhibitory small molecules at sites away from the active site by reversible disulfide bond formation. We screened a library of 10,000 thiol-containing compounds against accessible cysteines of two members of the caspase family of proteases, caspase-3 and -7. We discovered a previously unreported and conserved allosteric site in a deep cavity at the dimer interface 14 A from the active site. This site contains a natural cysteine that, when disulfide-bonded with either of two specific compounds, inactivates these proteases. The allosteric site is functionally coupled to the active site, such that binding of the compounds at the allosteric site prevents peptide binding at the active site. The x-ray crystal structures of caspase-7 bound by either compound demonstrates that they inhibit caspase-7 by trapping a zymogen-like conformation. This approach may be useful to identify new allosteric sites from natural or engineered cysteines, to study allosteric transitions in proteins, and to nucleate drug discovery efforts.
Organizational Affiliation:
Sunesis Pharmaceuticals, Inc., 341 Oyster Point Boulevard, South San Francisco, CA 94080, USA.