High-resolution structures of single-metal-substituted concanavalin A: the Co,Ca-protein at 1.6 A and the Ni,Ca-protein at 2.0 A.
Emmerich, C., Helliwell, J.R., Redshaw, M., Naismith, J.H., Harrop, S.J., Raftery, J., Kalb, A.J., Yariv, J., Dauter, Z., Wilson, K.S.(1994) Acta Crystallogr D Biol Crystallogr 50: 749-756
- PubMed: 15299372
- DOI: https://doi.org/10.1107/S0907444994002143
- Primary Citation of Related Structures:
1SCR, 1SCS - PubMed Abstract:
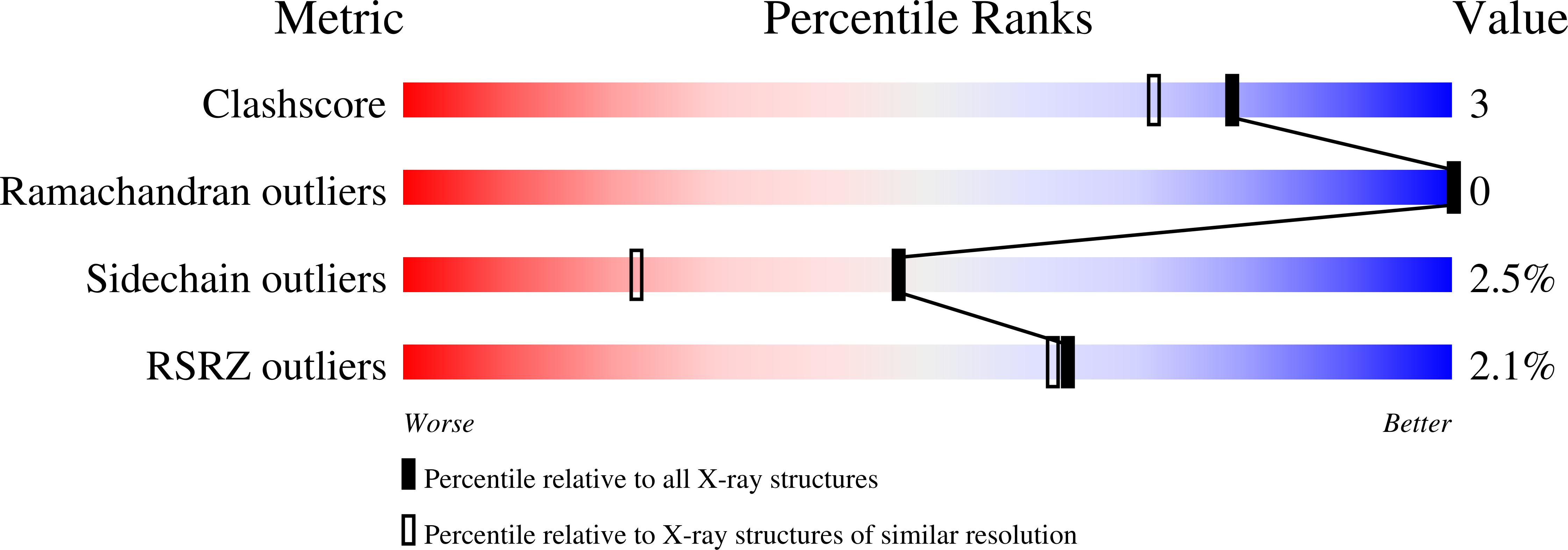
The molecular structures of cobalt- and nickel-substituted concanavalin A have been refined at 1.6 and 2.0 A resolution, respectively. Both metal derivatives crystallize in space group I222 with approximate cell dimensions a = 89, b = 87 and c = 63 A and one monomer in the asymmetric unit. The final R factor for Co-substituted concanavalin A is 17.8% for 29 211 reflections with F > 1.0sigma(F) between 8.0 and 1.6 A. For Ni-substituted concanavalin A the final R factor is 15.9% for 16 128 reflections with F > 1.0sigma(F) between 8.0 and 2.0 A resolution. Both structures contain a transition-metal binding site and a calcium-binding site but, unlike Cd-substituted concanavalin A, do not have a third metal-binding site. The Co-substituted concanavalin A structure diffracts to the highest resolution of any concanavalin A structure reported to date. A comparison of the structures of Ni-, Co-, Cd-substituted and native concanavalin A gives an indication of coordinate errors, which is a useful baseline for comparisons with saccharide complexes of concanavalin A described in other work. We also give a detailed account of multiple conformations which were found for five side-chain residues.
Organizational Affiliation:
Department of Chemistry, University of Manchester, England.