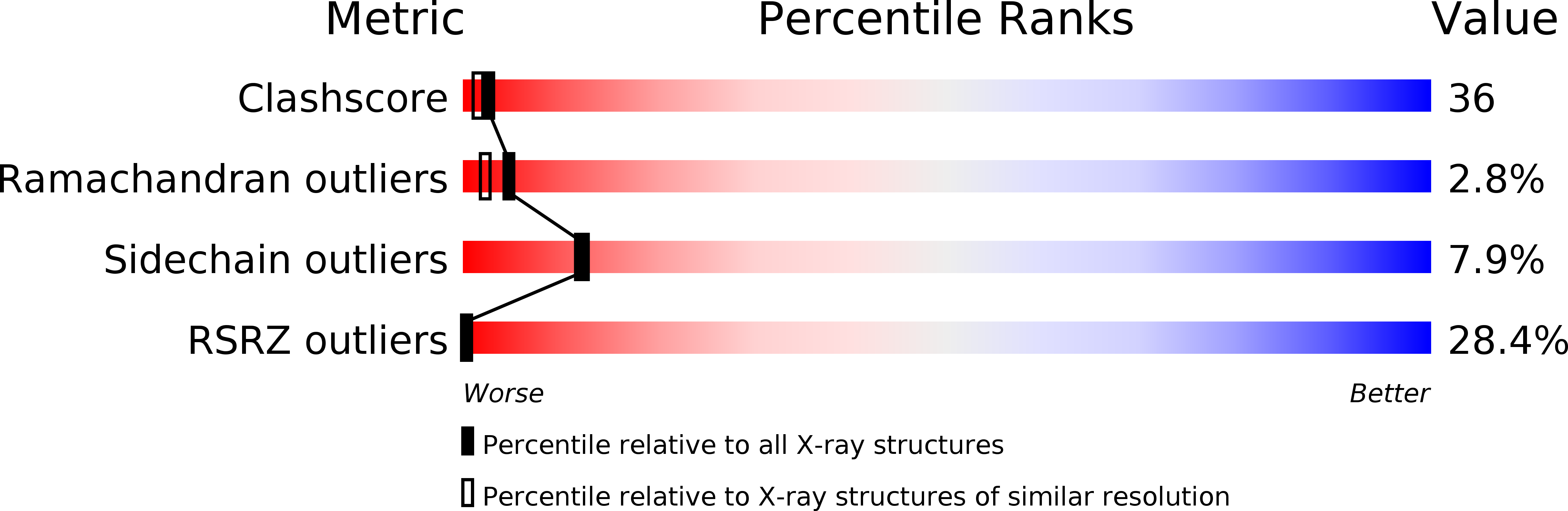
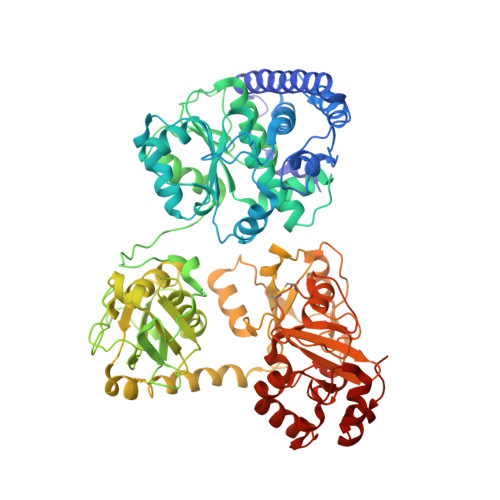
A functional Ni-Ni-[4Fe-4S] cluster in the monomeric acetyl-CoA synthase from Carboxydothermus hydrogenoformans
Svetlitchnyi, V., Dobbek, H., Meyer-Klaucke, W., Meins, T., Thiele, B., Rmer, P., Huber, R., Meyer, O.(2004) Proc Natl Acad Sci U S A 101: 446-451
- PubMed: 14699043
- DOI: https://doi.org/10.1073/pnas.0304262101
- Primary Citation of Related Structures:
1RU3 - PubMed Abstract:
In anaerobic microorganisms employing the acetyl-CoA pathway, acetyl-CoA synthase (ACS) and CO dehydrogenase (CODH) form a complex (ACS/CODH) that catalyzes the synthesis of acetyl-CoA from CO, a methyl group, and CoA. Previously, a [4Fe-4S] cubane bridged to a copper-nickel binuclear site (active site cluster A of the ACS component) was identified in the ACS(Mt)/CODH(Mt) from Moorella thermoacetica whereas another study revealed a nickel-nickel site in the open form of ACS(Mt), and a zink-nickel site in the closed form. The ACS(Ch) of the hydrogenogenic bacterium Carboxydothermus hydrogenoformans was found to exist as an 82.2-kDa monomer as well as in a 1:1 molar complex with the 73.3-kDa CODHIII(Ch). Homogeneous ACS(Ch) and ACS(Ch)/CODHIII(Ch) catalyzed the exchange between [1-(14)C]acetyl-CoA and (12)CO with specific activities of 2.4 or 5.9 micromol of CO per min per mg, respectively, at 70 degrees C and pH 6.0. They also catalyzed the synthesis of acetyl-CoA from CO, methylcobalamin, corrinoid iron-sulfur protein, and CoA with specific activities of 0.14 or 0.91 micromol of acetyl-CoA formed per min per mg, respectively, at 70 degrees C and pH 7.3. The functional cluster A of ACS(Ch) contains a Ni-Ni-[4Fe-4S] site, in which the positions proximal and distal to the cubane are occupied by Ni ions. This result is apparent from a positive correlation of the Ni contents and negative correlations of the Cu or Zn contents with the acetyl-CoA/CO exchange activities of different preparations of monomeric ACS(Ch), a 2.2-A crystal structure of the dithionite-reduced monomer in an open conformation, and x-ray absorption spectroscopy.
Organizational Affiliation:
Lehrstuhl für Mikrobiologie, Universität Bayreuth, D-95440 Bayreuth, Germany. vitali.svetlitchnyi@uni-bayreuth.de