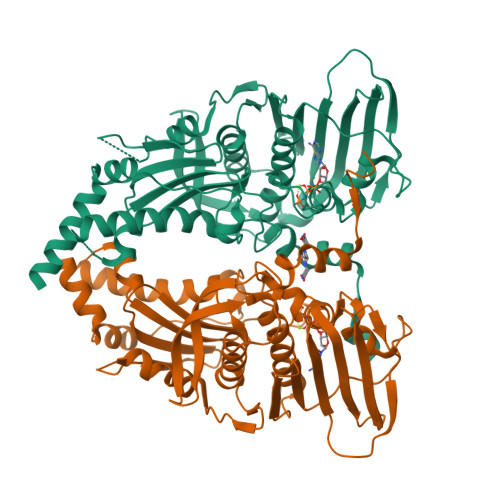
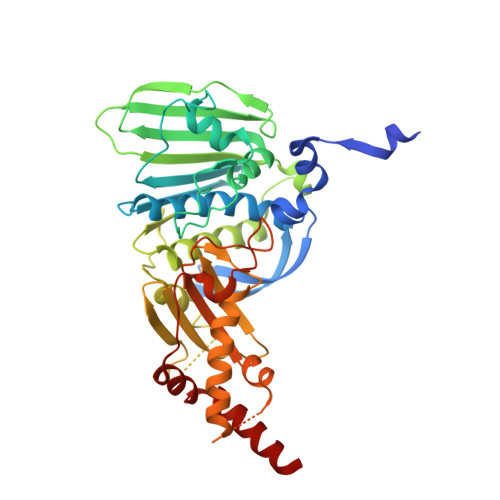
Structure of the topoisomerase II ATPase region and its mechanism of inhibition by the chemotherapeutic agent ICRF-187
Classen, S., Olland, S., Berger, J.M.(2003) Proc Natl Acad Sci U S A 100: 10629-10634
- PubMed: 12963818
- DOI: https://doi.org/10.1073/pnas.1832879100
- Primary Citation of Related Structures:
1PVG, 1QZR - PubMed Abstract:
Type IIA topoisomerases both manage the topological state of chromosomal DNA and are the targets of a variety of clinical agents. Bisdioxopiperazines are anticancer agents that associate with ATP-bound eukaryotic topoisomerase II (topo II) and convert the enzyme into an inactive, salt-stable clamp around DNA. To better understand both topo II and bisdioxopiperazine function, we determined the structures of the adenosine 5'-[beta,gamma-imino]-triphosphate-bound yeast topo II ATPase region (ScT2-ATPase) alone and complexed with the bisdioxopiperazine ICRF-187. The drug-free form of the protein is similar in overall fold to the equivalent region of bacterial gyrase but unexpectedly displays significant conformational differences. The ternary drug-bound complex reveals that ICRF-187 acts by an unusual mechanism of inhibition in which the drug does not compete for the ATP-binding pocket, but bridges and stabilizes a transient dimer interface between two ATPase protomers. Our data explain why bisdioxopiperazines target ATP-bound topo II, provide a structural rationale for the effects of certain drug-resistance mutations, and point to regions of bisdioxopiperazines that might be modified to improve or alter drug specificity.
Organizational Affiliation:
Department of Molecular and Cell Biology, 237 Hildebrand Hall, University of California, Berkeley, CA 94720-3206, USA.