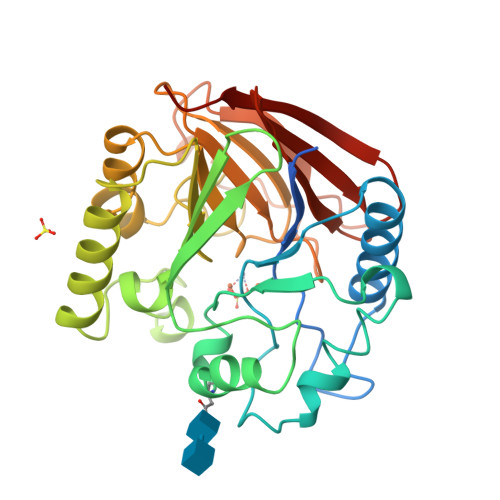
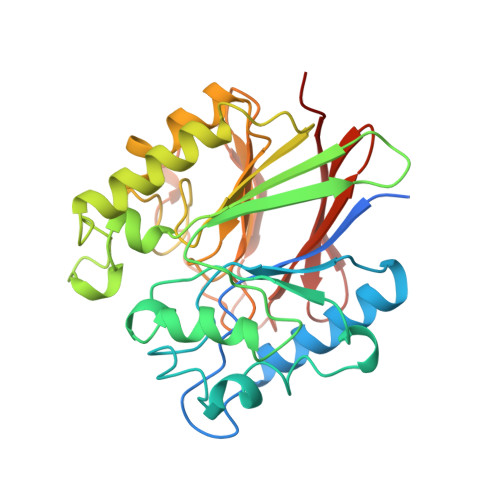
Three-dimensional structure of a mammalian purple acid phosphatase at 2.2 A resolution with a mu-(hydr)oxo bridged di-iron center.
Lindqvist, Y., Johansson, E., Kaija, H., Vihko, P., Schneider, G.(1999) J Mol Biology 291: 135-147
- PubMed: 10438611
- DOI: https://doi.org/10.1006/jmbi.1999.2962
- Primary Citation of Related Structures:
1QHW - PubMed Abstract:
The crystal structure of purple acid phosphatase from rat bone has been determined by molecular replacement and the structure has been refined to 2.2 A resolution to an R -factor of 21.3 % (R -free 26.5 %). The core of the enzyme consists of two seven-stranded mixed beta-sheets, with each sheet flanked by solvent-exposed alpha-helices on one side. The two sheets pack towards each other forming a beta-sandwich. The di-iron center, located at the bottom of the active-site pocket at one edge of the beta-sandwich, contains a mu-hydroxo or mu-oxo bridge and both metal ions are observed in an almost perfect octahedral coordination geometry. The electron density map indicates that a mu-(hydr)oxo bridge is found in the metal center and that at least one solvent molecule is located in the first coordination sphere of one of the metal ions. The crystallographic study of rat purple acid phosphatase reveals that the mammalian enzymes are very similar in overall structure to the plant enzymes in spite of only 18 % overall sequence identity. In particular, coordination and geometry of the iron cluster is preserved in both enzymes and comparison of the active-sites suggests a common mechanism for the mammalian and plant enzymes. However, significant differences are found in the architecture of the substrate binding pocket.
Organizational Affiliation:
Department of Medical Biochemistry & Biophysics, Karolinska Institutet, Stockholm, S-171 77, Sweden. ylva@alfa.mbb.ki.se