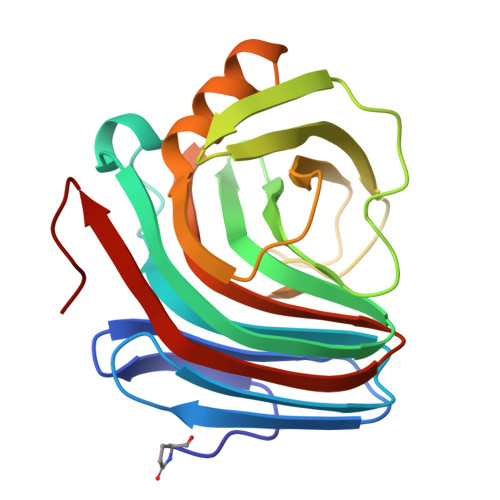
Catalysis and specificity in enzymatic glycoside hydrolysis: a 2,5B conformation for the glycosyl-enzyme intermediate revealed by the structure of the Bacillus agaradhaerens family 11 xylanase.
Sabini, E., Sulzenbacher, G., Dauter, M., Dauter, Z., Jorgensen, P.L., Schulein, M., Dupont, C., Davies, G.J., Wilson, K.S.(1999) Chem Biol 6: 483-492
- PubMed: 10381409
- DOI: https://doi.org/10.1016/s1074-5521(99)80066-0
- Primary Citation of Related Structures:
1H4G, 1H4H, 1QH6, 1QH7 - PubMed Abstract:
The enzymatic hydrolysis of glycosides involves the formation and subsequent breakdown of a covalent glycosyl-enzyme intermediate via oxocarbenium-ion-like transition states. The covalent intermediate may be trapped on-enzyme using 2-fluoro-substituted glycosides, which provide details of the intermediate conformation and noncovalent interactions between enzyme and oligosaccharide. Xylanases are important in industrial applications - in the pulp and paper industry, pretreating wood with xylanases decreases the amount of chlorine-containing chemicals used. Xylanases are structurally similar to cellulases but differ in their specificity for xylose-based, versus glucose-based, substrates. The structure of the family 11 xylanase, Xyl11, from Bacillus agaradhaerens has been solved using X-ray crystallography in both native and xylobiosyl-enzyme intermediate forms at 1.78 A and 2.0 A resolution, respectively. The covalent glycosyl-enzyme intermediate has been trapped using a 2-fluoro-2-deoxy substrate with a good leaving group. Unlike covalent intermediate structures for glycoside hydrolases from other families, the covalent glycosyl-enzyme intermediate in family 11 adopts an unusual 2,5B conformation. The 2,5B conformation found for the alpha-linked xylobiosyl-enzyme intermediate of Xyl11, unlike the 4C1 chair conformation observed for other systems, is consistent with the stereochemical constraints required of the oxocarbenium-ion-like transition state. Comparison of the Xyl11 covalent glycosyl-enzyme intermediate with the equivalent structure for the related family 12 endoglucanase, CelB, from Streptomyces lividans reveals the likely determinants for substrate specificity in this clan of glycoside hydrolases.
Organizational Affiliation:
Structural Biology Laboratory, Department of Chemistry, University of York, Heslington, York, Y010 5DD, UK.