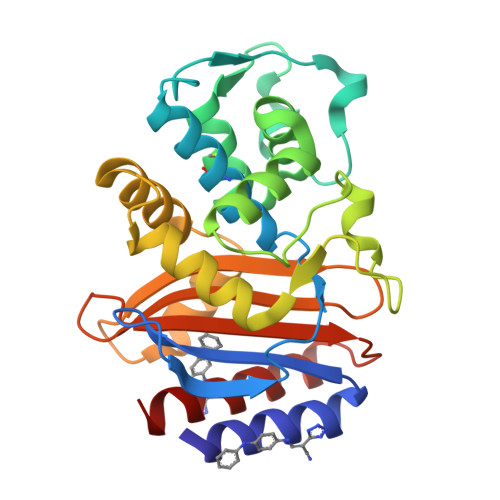
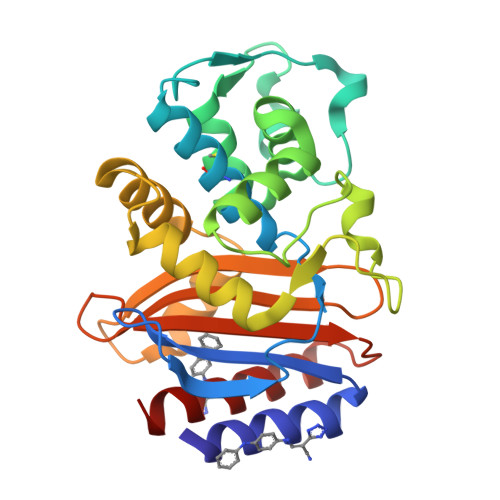
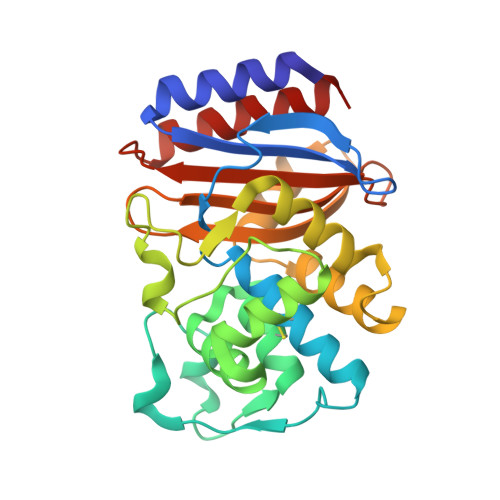
Allosteric inhibition through core disruption.
Horn, J.R., Shoichet, B.K.(2004) J Mol Biol 336: 1283-1291
- PubMed: 15037085
- DOI: https://doi.org/10.1016/j.jmb.2003.12.068
- Primary Citation of Related Structures:
1PZO, 1PZP - PubMed Abstract:
Although inhibitors typically bind pre-formed sites on proteins, it is theoretically possible to inhibit by disrupting the folded structure of a protein or, in the limit, to bind preferentially to the unfolded state. Equilibria defining how such molecules act are well understood, but structural models for such binding are unknown. Two novel inhibitors of beta-lactamase were found to destabilize the enzyme at high temperatures, but at lower temperatures showed no preference for destabilized mutant enzymes versus stabilized mutants. X-ray crystal structures showed that both inhibitors bound to a cryptic site in beta-lactamase, which the inhibitors themselves created by forcing apart helixes 11 and 12. This opened up a portion of the hydrophobic core of the protein, into which these two inhibitors bind. Although this binding site is 16 A from the center of the active site, the conformational changes were transmitted through a sequence of linked motions to a key catalytic residue, Arg244, which in the complex adopts conformations very different from those in catalytically competent enzyme conformations. These structures offer a detailed view of what has heretofore been a theoretical construct, and suggest the possibility for further design against this novel site.
Organizational Affiliation:
Drug Discovery Program, Department of Molecular Pharmacology and Biological Chemistry, Northwestern University School of Medicine, 303 East Chicago Ave, Chicago, IL 60611-3008, USA.