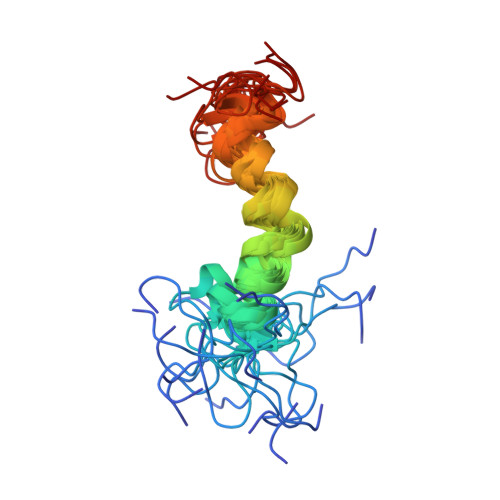
Solution structure of a polypeptide containing four heptad repeat units from a merozoite surface antigen of Plasmodium falciparum.
Mulhern, T.D., Howlett, G.J., Reid, G.E., Simpson, R.J., McColl, D.J., Anders, R.F., Norton, R.S.(1995) Biochemistry 34: 3479-3491
- PubMed: 7893643
- DOI: https://doi.org/10.1021/bi00011a001
- Primary Citation of Related Structures:
1PSM - PubMed Abstract:
The Plasmodium falciparum antigen SPAM (secreted polymorphic antigen associated with merozoites) contains an unusual set of heptad repeat units with alanine at the a and d positions. Twelve heptads with the consensus sequence AXXAXXX occur in three blocks of four, linked by short nonrepetitive sequences. A 38-residue polypeptide comprising the first block of four heptad units and five flanking residues at either end, SPAM-H1, has been synthesized and its structure in aqueous solution determined from 1H NMR data. Sedimentation equilibrium showed the peptide to be monomeric in aqueous solution. Its structure was determined from 1H NMR-derived distance and dihedral angle constraints by using distance geometry calculations, restrained simulated annealing, and conjugate gradient energy minimization in the CHARMm force field. The polypeptide contains an alpha-helix extending from Ser10 (position e of the first heptad) to at least Lys32 (position f of the fourth heptad) and possibly as far as Val35. The helix is bent, partly as result of a kink around residues 19-20. The conformations of the nine N-terminal residues and the six C-terminal residues are not well defined by the NMR data. The rms deviation from the average of the 20 best structures over the well-defined region (residues 11-31, which have backbone angular order parameters > 0.8) was 1.56 A for backbone heavy atoms (N, C alpha, and C) and 2.12 A for all heavy atoms. 2H2O exchange experiments identified slowly exchanging amide protons near the C-terminus and the last two turns of the helix. The unusual stability of the C-terminus reflects the presence of a new C-capping motif, which may involve the side chain of an asparagine in a position external to the C-cap residue. Possible interactions of the H1 sequence with the other two heptad repeat units in the intact merozoite antigen are discussed.
Organizational Affiliation:
NMR Laboratory, Biomolecular Research Institute, Parkville, Victoria, Australia.