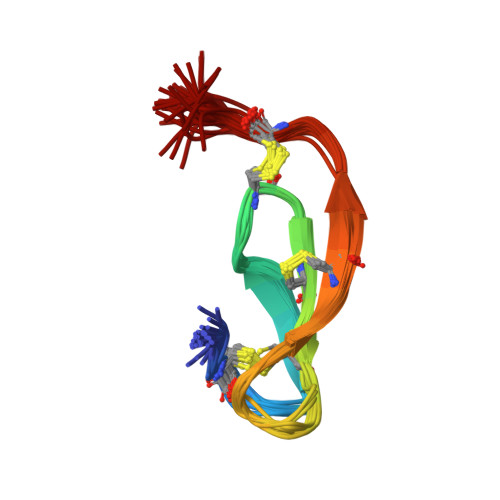
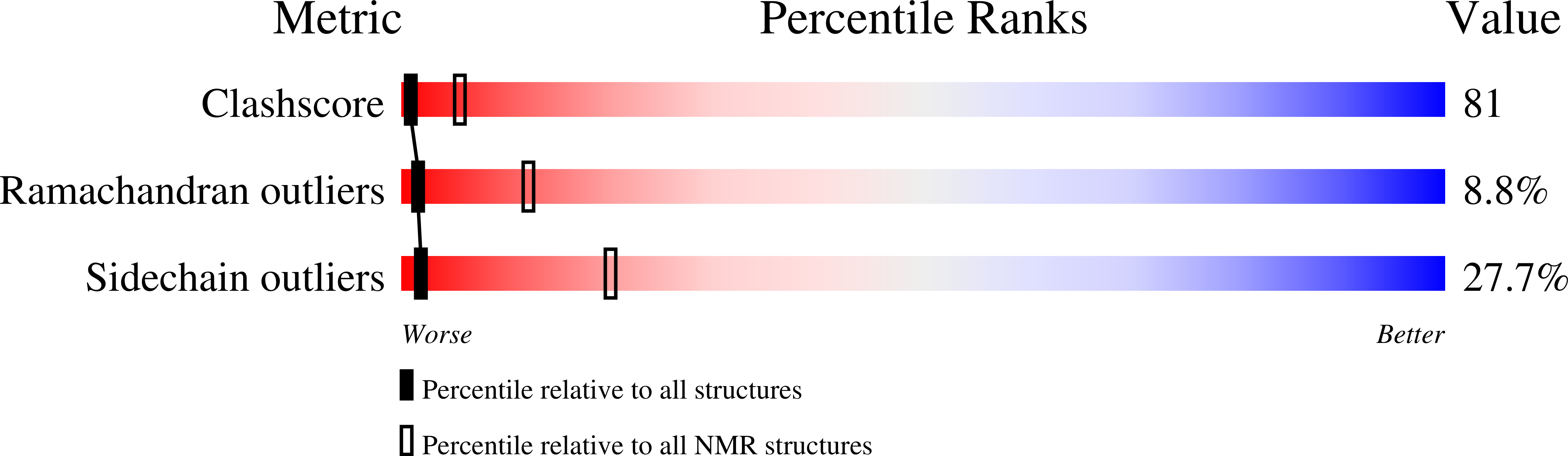Solution structure of PMP-C: a new fold in the group of small serine proteinase inhibitors.
Mer, G., Hietter, H., Kellenberger, C., Renatus, M., Luu, B., Lefevre, J.F.(1996) J Mol Biology 258: 158-171
- PubMed: 8613985
- DOI: https://doi.org/10.1006/jmbi.1996.0240
- Primary Citation of Related Structures:
1PMC - PubMed Abstract:
The solution structure and the disulfide pairings of a 36-residue proteinase inhibitor isolated from the insect Locusta migratoria have been determined using NMR spectroscopy and simulated annealing calculations. The peptide, termed PMP-C, was previously shown to inhibit bovine alpha-chymotrypsin as well as human leukocyte elastase, and was also found to block high-voltage-activated Ca2+ currents in rat sensory neurones. PMP-C has a prolate ellipsoid shape and adopts a tertiary fold hitherto unobserved in the large group of small "canonical" proteinase inhibitors. The over-all fold consists mainly of three strands arranged in a right-handed twisted, antiparallel, beta-sheet that demarcates a cavity, together with a linear amino-terminal segment oriented almost perpendicular to the three strands of the beta-sheet. Inside the cavity a phenyl ring constitutes the centre of a hydrophobic core. The proteinase binding loop is located in the carboxy-terminal part of the molecule, between two cysteine residues involved in disulfide bridges. Its conformation resembles that found in other small canonical proteinase inhibitors. A comparison of PMP-C structure with the recently published solution structure of the related peptide PMP-D2 shows that the most significant differences are complementary changes involved in the stabilization of similar folds. This comparison led us to review the structure of PMP-D2 and to identify two salt bridges in PMP-D2.
Organizational Affiliation:
CNRS-UPR 9003, Ecole Supérieure de Biotechnologie de Strasbourg, Illkirch-Graffenstaden, France.