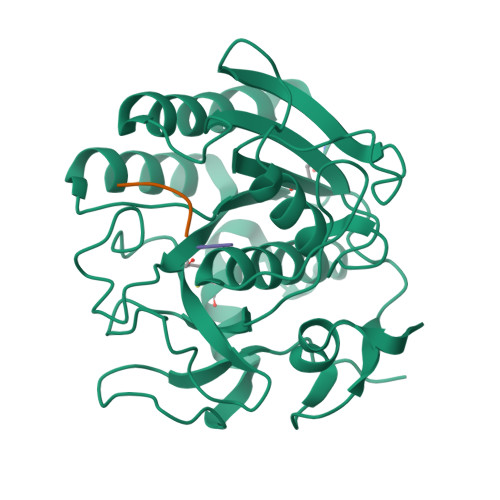
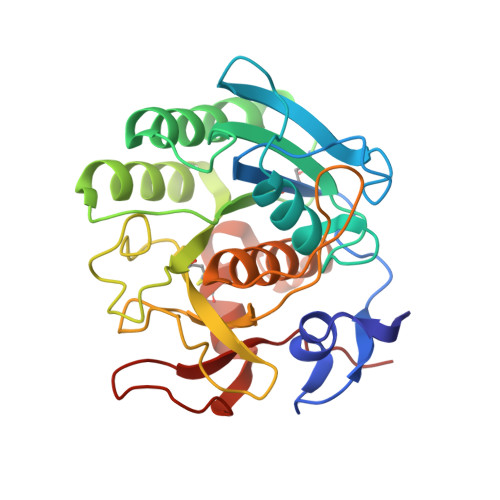
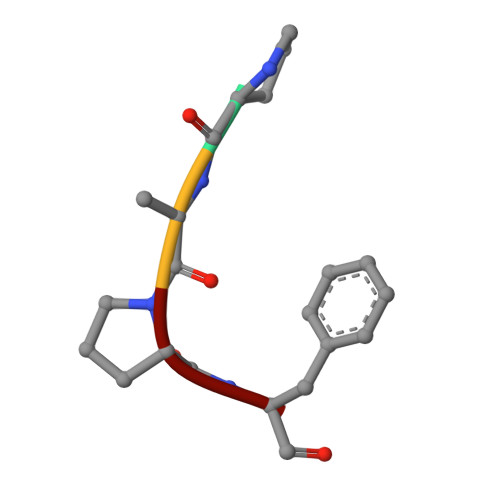
Structure of the complex of proteinase K with a substrate analogue hexapeptide inhibitor at 2.2-A resolution.
Betzel, C., Singh, T.P., Visanji, M., Peters, K., Fittkau, S., Saenger, W., Wilson, K.S.(1993) J Biological Chem 268: 15854-15858
- PubMed: 8340410
- Primary Citation of Related Structures:
1PEK - PubMed Abstract:
The crystal structure of a transition state/product complex formed by the interaction between proteinase K and the substrate analogue N-Ac-L-Pro-L-Ala-L-Pro-L-Phe-D-Ala-L-Ala-NH2 has been determined at a resolution of 2.2 A and refined to an R-factor of 0.165 for 12,725 reflections. The inhibitor forms a stable complex through a series of hydrogen bonds with protein atoms and water molecules. The inhibitor is hydrolyzed between Phe 4I and D-Ala5I (I indicates inhibitor). The two fragments are separated by a distance of 3.07 A between the carbonyl carbon and the main chain nitrogen. Both fragments remain bound to the protein. The N-terminal fragment occupies subsites S5 to S1, whereas the C-terminal part is bound in S1' and S2', the first time that electron density for a substrate analogue has been observed in the P1' and P2' sites of a subtilisin-like enzyme. The flexible segments of the substrate recognition sites Gly100-Tyr104 and Ser132-Gly136 move appreciably to accommodate the inhibitor. Biochemical results indicate an inhibition by this specifically designed peptide of 95%.
Organizational Affiliation:
European Molecular Biology Laboratory, Hamburg, Germany.