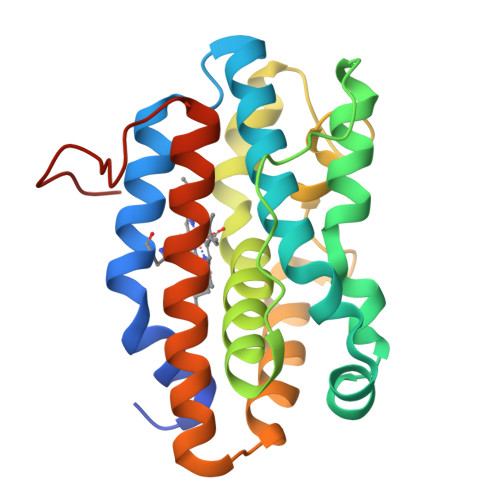
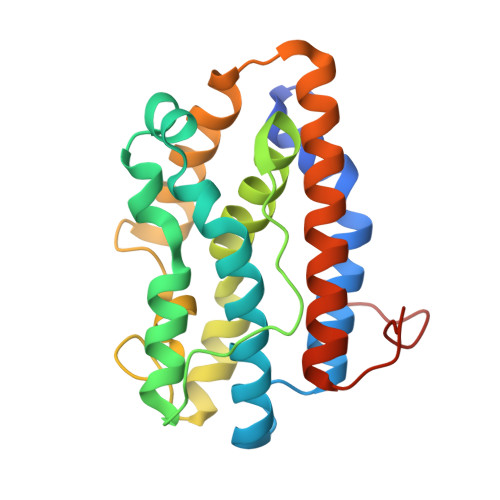
Crystal structures of the NO- and CO-bound heme oxygenase from Neisseriae meningitidis. Implications for O2 activation
Friedman, J., Lad, L., Deshmukh, R., Li, H., Wilks, A., Poulos, T.L.(2003) J Biological Chem 278: 34654-34659
- PubMed: 12819228
- DOI: https://doi.org/10.1074/jbc.M302985200
- Primary Citation of Related Structures:
1P3T, 1P3U, 1P3V - PubMed Abstract:
Heme oxygenases catalyze the oxidation of heme to biliverdin, carbon monoxide, and free iron while playing a critical role in mammalian heme homeostasis. Pathogenic bacteria such as Neisseriae meningitidis also produce heme oxygenase as part of a mechanism to mine host iron. The key step in heme oxidation is the regioselective oxidation of the heme alpha-meso-carbon by an activated Fe(III)-OOH complex. The structures of various diatomic ligands bound to the heme iron can mimic the dioxygen complex and provide important insights on the mechanism of O2 activation. Here we report the crystal structures of N. meningitidis heme oxygenase (nm-HO) in the Fe(II), Fe(II)-CO, and Fe(II)-NO states and compare these to the NO complex of human heme oxygenase-1 (Lad, L., Wang, J., Li, H., Friedman, J., Bhaskar, B., Ortiz de Montellano, P. R., and Poulos, T. L. (2003) J. Mol. Biol. 330, 527-538). Coordination of NO or CO results in a reorientation of Arg-77 that enables Arg-77 to participate in an active site H-bonded network involving a series of water molecules. One of these water molecules directly H-bonds to the Fe(II)-linked ligand and very likely serves as the proton source required for oxygen activation. Although the active site residues differ between nm-HO and human HO-1, the close similarity in the H-bonded water network suggests a common mechanism shared by all heme oxygenases.
Organizational Affiliation:
Department of Molecular Biology and Biochemistry, and Program in Macromolecular Structure, University of California, Irvine, California 92697, USA.