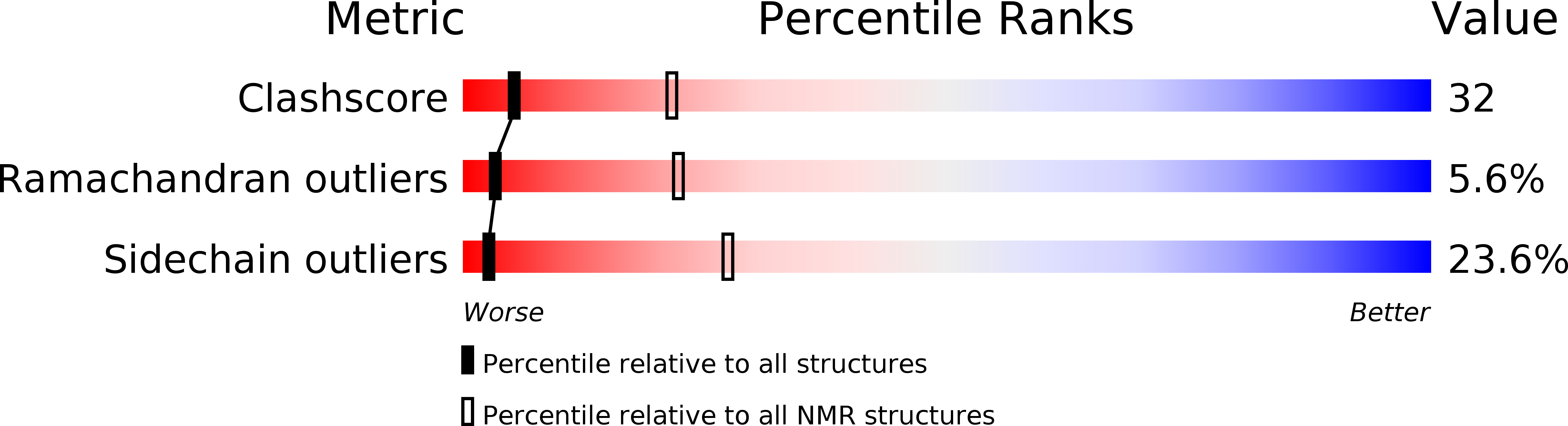NMR studies of the interaction of tryparedoxin with redox-inactive substrate homologues.
Krumme, D., Budde, H., Hecht, H.J., Menge, U., Ohlenschlager, O., Ross, A., Wissing, J., Wray, V., Flohe, L.(2003) Biochemistry 42: 14720-14728
- PubMed: 14674746
- DOI: https://doi.org/10.1021/bi030112d
- Primary Citation of Related Structures:
1OKD - PubMed Abstract:
Tryparedoxins (TXNs) are trypanothione-dependent peroxiredoxin oxidoreductases involved in hydroperoxide detoxification that have been shown to determine virulence in trypanosomatids. The structure of (15)N,(13)C-doubly-labeled, C-terminally-His-tagged tryparedoxin 1 from Crithidia fasciculata (Cf TXN1) was elucidated by three-dimensional NMR spectroscopy. Global folding was found to be similar to the crystal structure, but regions near the active site, especially the onset of helix alpha1 with the redox-active Cys 43 and helix alpha2 relevant to substrate binding, were less well defined in solution. The redox-inactive inhibitory substrate analogue N(1),N(8)-bis(ophthalmyl)spermidine was used to study the substrate/TXN interaction by two-dimensional (1)H,(15)N NMR spectroscopy. The NMR data complemented by molecular modeling revealed several alternative modes of ligand binding. The results confirm and extend the concept of TXN action and specificity derived from X-ray analysis and site-directed mutagenesis and thus improve the rational basis for inhibitor design.
Organizational Affiliation:
Department of Structural Biology, German Research Centre for Biotechnology, University of Braunschweig.