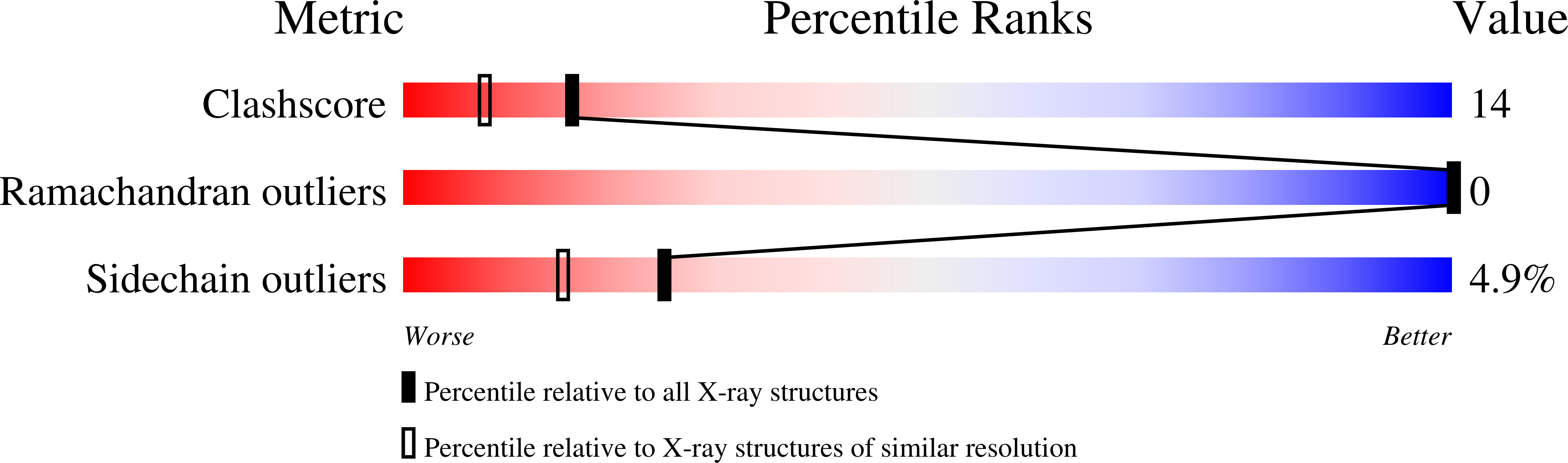Introduction and characterization of a functionally linked metal ion binding site at the exposed heme edge of myoglobin
Hunter, C.L., Maurus, R., Mauk, M.R., Lee, H., Raven, E.L., Tong, H., Nguyen, N., Smith, M., Brayer, G.D., Mauk, A.G.(2003) Proc Natl Acad Sci U S A 100: 3647-3652
- PubMed: 12644706
- DOI: https://doi.org/10.1073/pnas.0636702100
- Primary Citation of Related Structures:
1NZ2, 1NZ3, 1NZ4, 1NZ5 - PubMed Abstract:
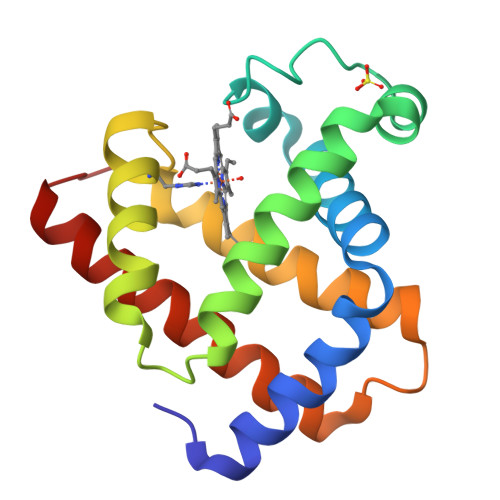
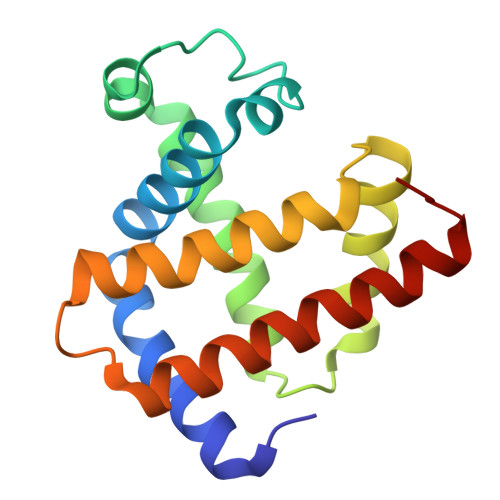
A binding site for metal ions has been created on the surface of horse heart myoglobin (Mb) near the heme 6-propionate group by replacing K45 and K63 with glutamyl residues. One-dimensional (1)H NMR spectroscopy indicates that Mn(2+) binds in the vicinity of the heme 6-propionate as anticipated, and potentiometric titrations establish that the affinity of the new site for Mn(2+) is 1.28(4) x 10(4) M(-1) (pH 6.96, ionic strength I = 17.2 microM, 25 degrees C). In addition, these substitutions lower the reduction potential of the protein and increase the pK(a) for the water molecule coordinated to the heme iron of metmyoglobin. The peroxidase [2,2'-azinobis(3-ethylbenzthiazoline-6-sulfonic acid), ABTS, as substrate] and the Mn(2+)-peroxidase activity of the variant are both increased approximately 3-fold. In contrast to wild-type Mb, both the affinity for azide and the midpoint potential of the variant are significantly influenced by the addition of Mn(2+). The structure of the variant has been determined by x-ray crystallography to define the coordination environment of bound Mn(2+) and Cd(2+). Although slight differences are observed between the geometry of the binding of the two metal ions, both are hexacoordinate, and neither involves coordination by E63.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology and Protein Engineering Network of Centres of Excellence, University of British Columbia, Vancouver, BC, Canada V6T 1Z3.