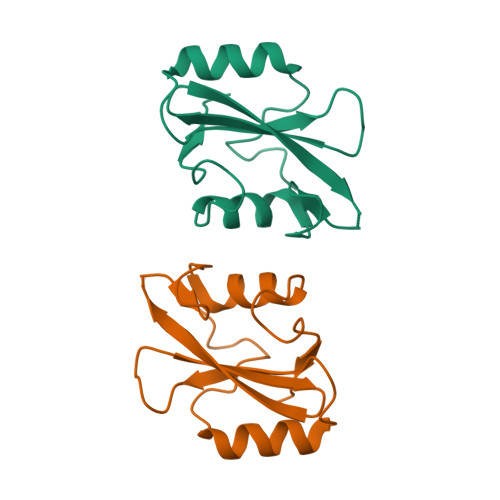
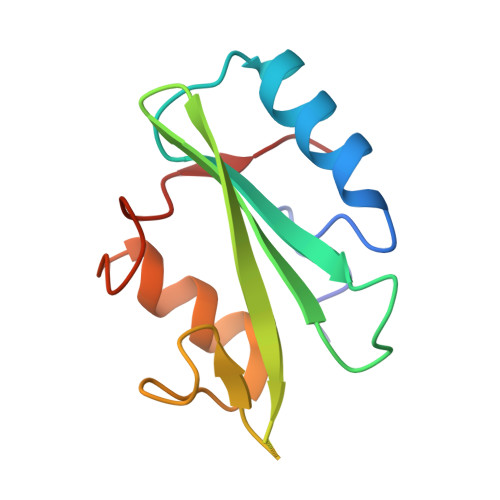
Structural basis for dimerization of the Grb10 Src homology 2 domain. Implications for ligand specificity.
Stein, E.G., Ghirlando, R., Hubbard, S.R.(2003) J Biological Chem 278: 13257-13264
- PubMed: 12551896
- DOI: https://doi.org/10.1074/jbc.M212026200
- Primary Citation of Related Structures:
1NRV - PubMed Abstract:
Grb7, Grb10, and Grb14 are members of a distinct family of adapter proteins that interact with various receptor tyrosine kinases upon receptor activation. Proteins in this family contain several modular signaling domains including a pleckstrin homology (PH) domain, a BPS (between PH and SH2) domain, and a C-terminal Src homology 2 (SH2) domain. Although SH2 domains are typically monomeric, we show that the Grb10 SH2 domain and also full-length Grb10 gamma are dimeric in solution under physiologic conditions. The crystal structure of the Grb10 SH2 domain at 1.65-A resolution reveals a non-covalent dimer whose interface comprises residues within and flanking the C-terminal alpha helix, which are conserved in the Grb7/Grb10/Grb14 family but not in other SH2 domains. Val-522 in the BG loop (BG3) and Asp-500 in the EF loop (EF1) are positioned to interfere with the binding of the P+3 residue of a phosphopeptide ligand. These structural features of the Grb10 SH2 domain will favor binding of dimeric, turn-containing phosphotyrosine sequences, such as the phosphorylated activation loops in the two beta subunits of the insulin and insulin-like growth factor-1 receptors. Moreover, the structure suggests the mechanism by which the Grb7 SH2 domain binds selectively to pTyr-1139 (pYVNQ) in Her2, which along with Grb7 is co-amplified in human breast cancers.
Organizational Affiliation:
Skirball Institute of Biomolecular Medicine and Department of Pharmacology, New York University School of Medicine, New York, New York 10016, USA.