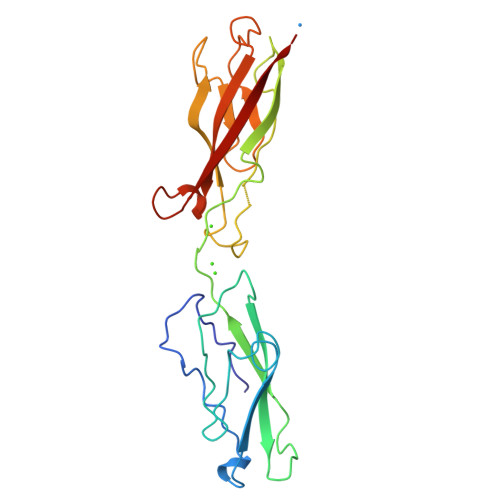
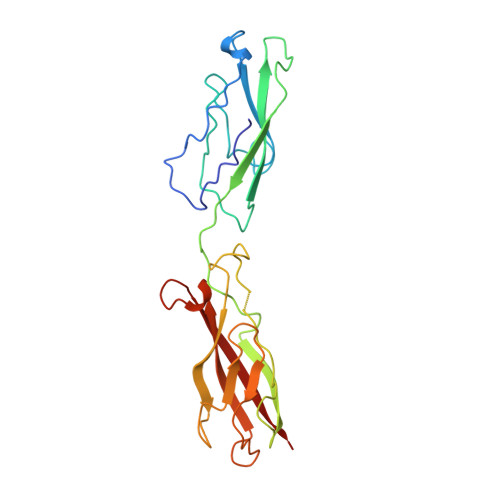
Structure-function analysis of cell adhesion by neural (N-) cadherin.
Tamura, K., Shan, W.S., Hendrickson, W.A., Colman, D.R., Shapiro, L.(1998) Neuron 20: 1153-1163
- PubMed: 9655503
- DOI: https://doi.org/10.1016/s0896-6273(00)80496-1
- Primary Citation of Related Structures:
1NCJ - PubMed Abstract:
To investigate the possible biological function of the lateral "strand dimer" observed in crystal structures of a D1 domain extracellular fragment from N-cadherin, we have undertaken site-directed mutagenesis studies of this molecule. Mutation of most residues important in the strand dimer interface abolish the ability of N-cadherin to mediate cell adhesion. Mutation of an analogous central residue (Trp-2) in E-cadherin also abrogates the adhesive capacity of that molecule. We also determined the crystal structure of a Ca2+-complexed two-domain fragment from N-cadherin. This structure, like its E-cadherin counterpart, does not adopt the strand dimer conformation. This suggests the possibility that classical cadherins might stably exist in both dimeric and monomeric forms. Data from several laboratories imply that lateral dimerization or clustering of cadherins may increase their adhesivity. We suggest the possibility that the strand dimer may play a role in this activation.
Organizational Affiliation:
Brookdale Center for Developmental and Molecular Biology, Mount Sinai School of Medicine, New York, New York 10029, USA.