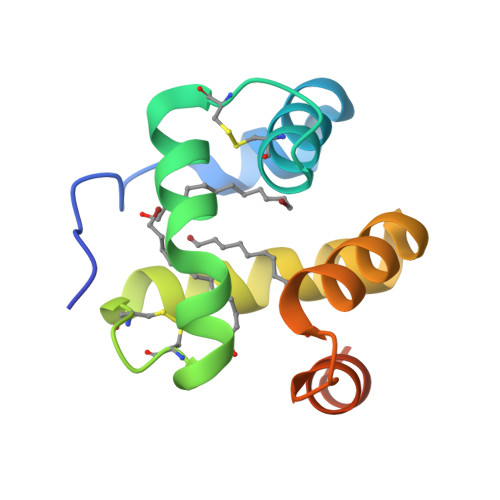
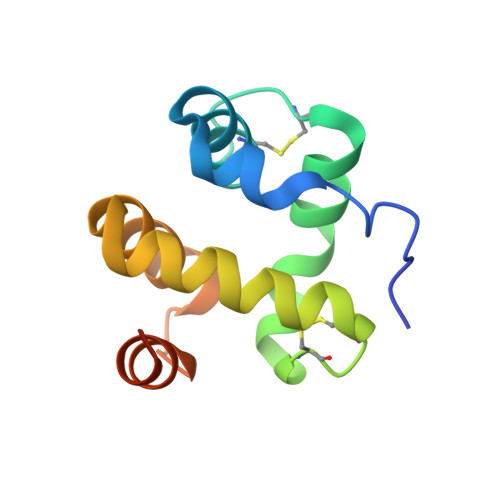
Moth chemosensory protein exhibits drastic conformational changes and cooperativity on ligand binding.
Campanacci, V., Lartigue, A., Hallberg, B.M., Jones, T.A., Giudici-Orticoni, M.T., Tegoni, M., Cambillau, C.(2003) Proc Natl Acad Sci U S A 100: 5069-5074
- PubMed: 12697900
- DOI: https://doi.org/10.1073/pnas.0836654100
- Primary Citation of Related Structures:
1N8U, 1N8V - PubMed Abstract:
Chemosensory proteins (CSPs) have been proposed to transport hydrophobic chemicals from air to olfactory or taste receptors. They have been isolated from several sensory organs of a wide range of insect species. The x-ray structure of CSPMbraA6, a 112-aa antennal protein from the moth Mamestra brassicae (Mbra), was shown to exhibit a novel type of alpha-helical fold. We have performed a structural and binding study of CSPMbraA6 to get some insights into its possible molecular function. Tryptophan fluorescence quenching demonstrates the ability of CSPMbraA6 to bind several types of semio-chemicals or surrogate ligands with microM K(d). Its crystal structure in complex with one of these compounds, 12-bromo-dodecanol, reveals extensive conformational changes on binding, resulting in the formation of a large cavity filled by three ligand molecules. Furthermore, binding cooperativity was demonstrated for some ligands, suggesting a stepwise binding. The peculiar rearrangement of CSPMbraA6 conformation and the cooperativity phenomenon might trigger the recognition of chemicals by receptors and induce subsequent signal transduction.
Organizational Affiliation:
Architecture et Fonction des Macromolécules Biologiques, Unité Mixte de Recherche 6098, Centre National de la Recherche Scientifique and Universités d'Aix-Marseille I & II, 31 Chemin J. Aiguier, 13402 Marseille Cedex 20, France.