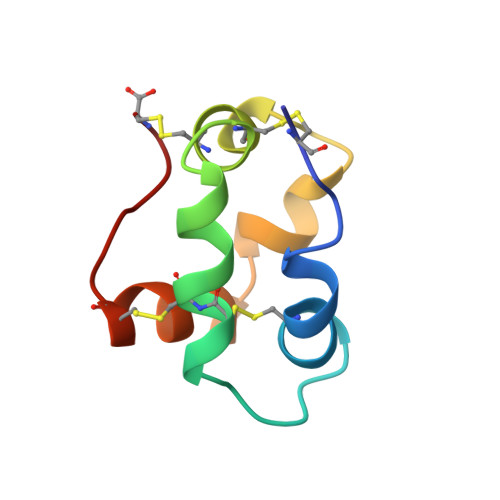
Refined solution structure of a liganded type 2 wheat nonspecific lipid transfer protein.
Pons, J.L., de Lamotte, F., Gautier, M.F., Delsuc, M.A.(2003) J Biological Chem 278: 14249-14256
- PubMed: 12525478
- DOI: https://doi.org/10.1074/jbc.M211683200
- Primary Citation of Related Structures:
1N89 - PubMed Abstract:
The refined structure of a wheat type 2 nonspecific lipid transfer protein (ns-LTP2) liganded with l-alpha-palmitoylphosphatidylglycerol has been determined by NMR. The (15)N-labeled protein was produced in Pichia pastoris. Physicochemical conditions and ligandation were intensively screened to obtain the best NMR spectra quality. This ns-LTP2 is a 67-residue globular protein with a diameter of about 30 A. The structure is composed of five helices forming a right superhelix. The protein presents an inner cavity, which has been measured at 341 A(3). All of the helices display hydrophobic side chains oriented toward the cavity. The phospholipid is found in this cavity. Its fatty acid chain is completely inserted in the protein, the l-alpha-palmitoylphosphatidylglycerol glycerol moiety being located on a positively charged pocket on the surface of the protein. The superhelix structure of the protein is coiled around the fatty acid chain. The overall structure shows similarities with ns-LTP1. Nevertheless, large three-dimensional structural discrepancies are observed for the H3 and H4 alpha-helices, the C-terminal region, and the last turn of the H2 helix. The lipid is orthogonal to the orientation observed in ns-LTP1. The volume of the hydrophobic cavity appears to be in the same range as the one of ns-LTP1, despite the fact that ns-LTP2 is shorter by 24 residues.
Organizational Affiliation:
Centre de Biochimie Structurale, INSERM, CNRS, Université Montpellier I, France.