Structure of mammalian protein geranylgeranyltransferase type-I
Taylor, J.S., Reid, T.S., Terry, K.L., Casey, P.J., Beese, L.S.(2003) EMBO J 22: 5963-5974
- PubMed: 14609943
- DOI: https://doi.org/10.1093/emboj/cdg571
- Primary Citation of Related Structures:
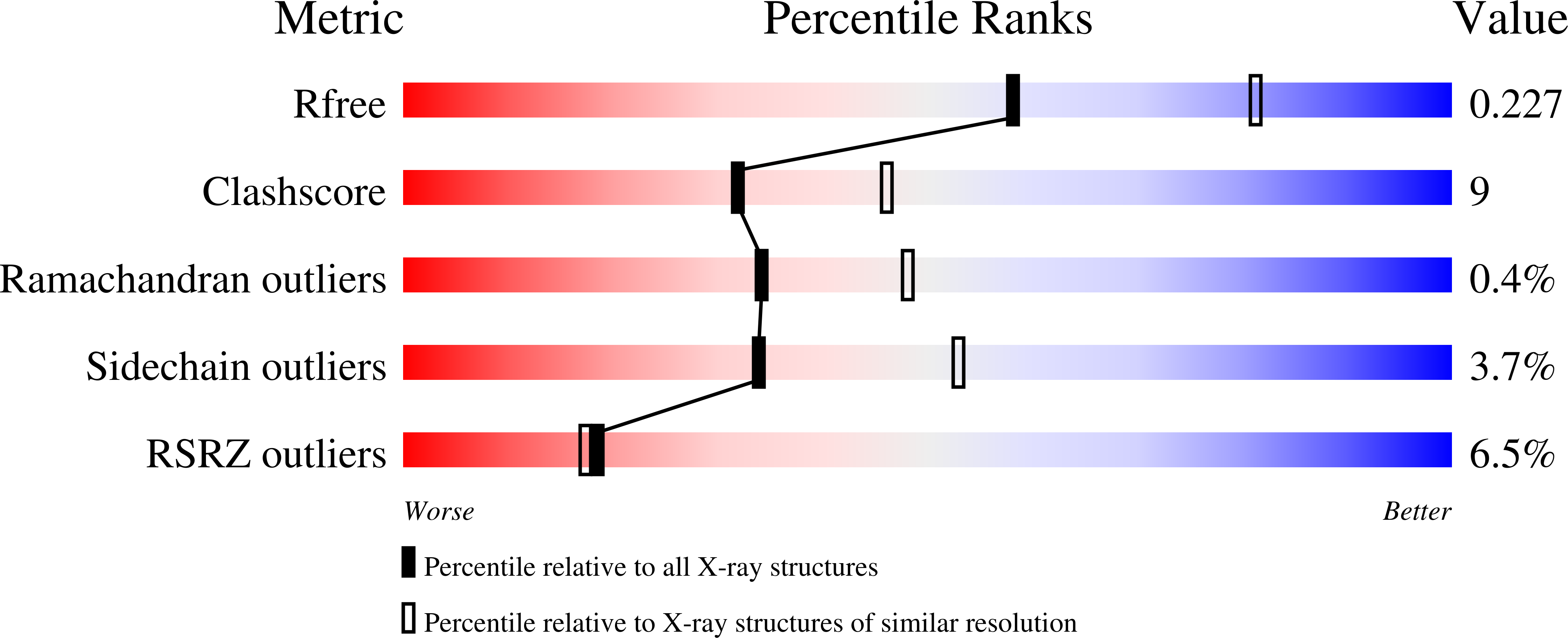
1N4P, 1N4Q, 1N4R, 1N4S - PubMed Abstract:
Protein geranylgeranyltransferase type-I (GGTase-I), one of two CaaX prenyltransferases, is an essential enzyme in eukaryotes. GGTase-I catalyzes C-terminal lipidation of >100 proteins, including many GTP- binding regulatory proteins. We present the first structural information for mammalian GGTase-I, including a series of substrate and product complexes that delineate the path of the chemical reaction. These structures reveal that all protein prenyltransferases share a common reaction mechanism and identify specific residues that play a dominant role in determining prenyl group specificity. This hypothesis was confirmed by converting farnesyltransferase (15-C prenyl substrate) into GGTase-I (20-C prenyl substrate) with a single point mutation. GGTase-I discriminates against farnesyl diphosphate (FPP) at the product turnover step through the inability of a 15-C FPP to displace the 20-C prenyl-peptide product. Understanding these key features of specificity is expected to contribute to optimization of anti-cancer and anti-parasite drugs.
Organizational Affiliation:
Department of Biochemistry, Duke University Medical Center, Durham, NC 27710, USA.