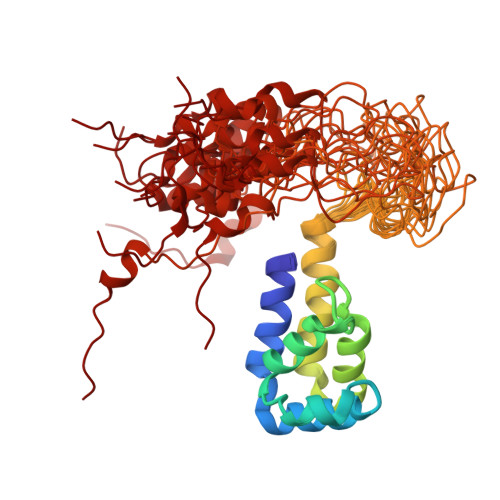
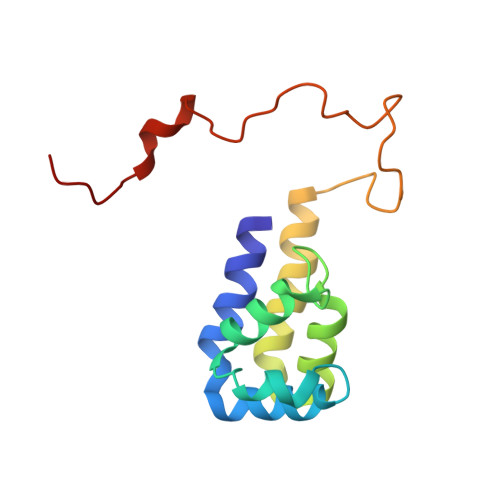
Recognition of ERK MAP Kinase by PEA-15 Reveals a Common Docking Site Within the Death Domain and Death Effector Domain
Hill, J.M., Vaidyanathan, H., Ramos, J.W., Ginsberg, M.H., Werner, M.H.(2002) EMBO J 21: 6494-6504
- PubMed: 12456656
- DOI: https://doi.org/10.1093/emboj/cdf641
- Primary Citation of Related Structures:
1N3K - PubMed Abstract:
PEA-15 is a multifunctional protein that modulates signaling pathways which control cell proliferation and cell death. In particular, PEA-15 regulates the actions of the ERK MAP kinase cascade by binding to ERK and altering its subcellular localization. The three-dimensional structure of PEA-15 has been determined using NMR spectroscopy and its interaction with ERK defined by characterization of mutants that modulate ERK function. PEA-15 is composed of an N-terminal death effector domain (DED) and a C-terminal tail of irregular structure. NMR 'footprinting' and mutagenesis identified elements of both the DED and tail that are required for ERK binding. Comparison of the DED-binding surface for ERK2 with the death domain (DD)-binding surface of Drosophila Tube revealed an unexpected similarity between the interaction modes of the DD and DED motifs in these proteins. Despite a lack of functional or sequence similarity between PEA-15 and Tube, these proteins utilize a common surface of the structurally similar DD and DED to recognize functionally diverse targets.
Organizational Affiliation:
Laboratory of Molecular Biophysics, The Rockefeller University, New York, NY 10021, USA.