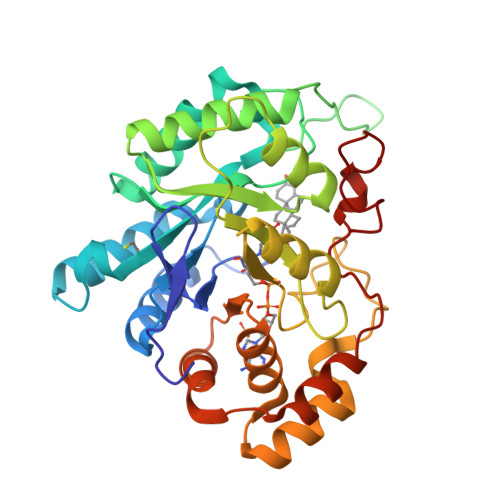
Human 20alpha-hydroxysteroid dehydrogenase: crystallographic and site-directed mutagenesis studies lead to the identification of an alternative binding site for C21-steroids.
Couture, J.F., Legrand, P., Cantin, L., Luu-The, V., Labrie, F., Breton, R.(2003) J Mol Biology 331: 593-604
- PubMed: 12899831
- DOI: https://doi.org/10.1016/s0022-2836(03)00762-9
- Primary Citation of Related Structures:
1MRQ - PubMed Abstract:
Human 20alpha-hydroxysteroid dehydrogenase (h20alpha-HSD; AKR1C1) catalyzes the transformation of progesterone (Prog) into 20alpha-hydroxy-progesterone (20alpha-OHProg). Although h20alpha-HSD shares 98% sequence identity with human type 3 3alpha-HSD (h3alpha-HSD3, AKR1C2), these two enzymes differ greatly in their activities. In order to explain these differences, we have solved the crystal structure of h20alpha-HSD in a ternary complex with NADP(+) and 20alpha-OHProg at 1.59A resolution. The steroid is stabilized by numerous hydrophobic interactions and a hydrogen bond between its O20 and the N(epsilon ) atom of His222. This new interaction prevents the formation of a hydrogen bond with the cofactor, as seen in h3alpha-HSD3 ternary complexes. By combining structural, direct mutagenesis and kinetic studies, we found that the H(222)I substitution decreases the K(m) value for the cofactor 95-fold. With these results, we hypothesize that the rotation of the lateral chain of His222 could be a mediating step between the transformation of Prog and the release of the cofactor. Moreover, crystal structure analysis and direct mutagenesis experiments lead us to identify a new residue involved in the binding of Prog. Indeed, the R(304)L substitution leads to a 65-fold decrease in the K(m) value for Prog reduction. We thus propose that Prog is maintained in a new steroid-binding site composed mainly of residues found in the carboxy-terminal region of the protein.
Organizational Affiliation:
Oncology and Molecular Endocrinology Research Center, Laval University Medical Center (CHUL) 2705 boul. Laurier, Ste-Foy, Qc., Canada G1V 4G2.