Structural and functional alterations of a colicin-resistant mutant of OmpF porin from Escherichia coli.
Jeanteur, D., Schirmer, T., Fourel, D., Simonet, V., Rummel, G., Widmer, C., Rosenbusch, J.P., Pattus, F., Pages, J.M.(1994) Proc Natl Acad Sci U S A 91: 10675-10679
- PubMed: 7524100
- DOI: https://doi.org/10.1073/pnas.91.22.10675
- Primary Citation of Related Structures:
1MPF - PubMed Abstract:
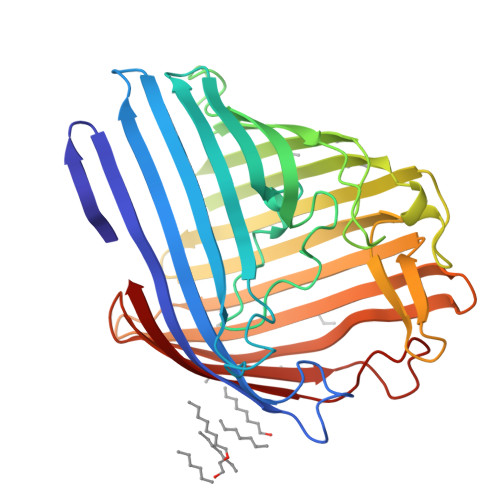
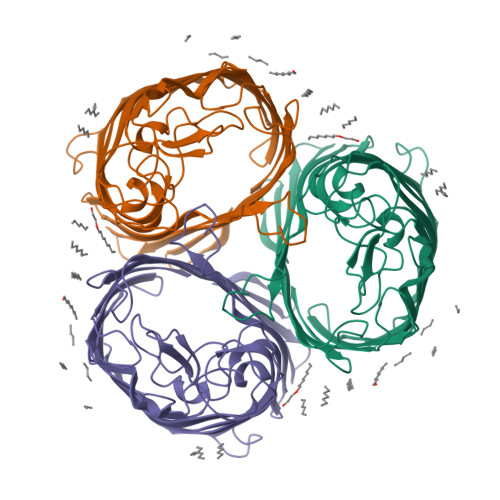
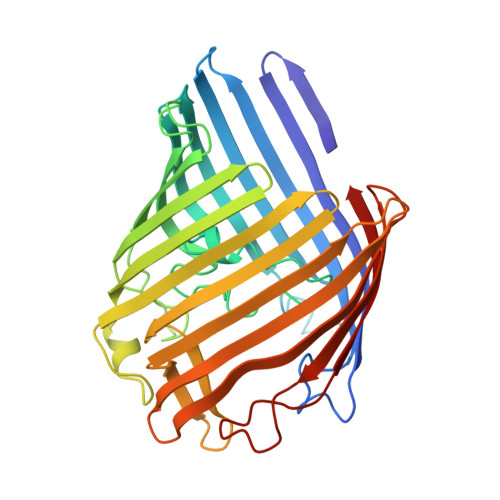
A strain of Escherichia coli, selected on the basis of its resistance to colicin N, reveals distinct structural and functional alterations in unspecific OmpF porin. A single mutation [Gly-119-->Asp (G119D)] was identified in the internal loop L3 that contributes critically to the formation of the construction inside the lumen of the pore. X-ray structure analysis to a resolution of 3.0 A reveals a locally altered peptide backbone, with the side chain of residue Asp-119 protruding into the channel, causing the area of the constriction (7 x 11 A in the wild type) to be subdivided into two intercommunicating subcompartments of 3-4 A in diameter. The functional consequences of this structural modification consist of a reduction of the channel conductance by about one-third, of altered ion selectivity and voltage gating, and of a decrease of permeation rates of various sugars by factors of 2-12. The structural modification of the mutant protein affects neither the beta-barrel structure nor those regions of the molecule that are exposed at the cell surface. Considering the colicin resistance of the mutant, it is inferred that in vivo, colicin N traverses the outer membrane through the porin channel or that the dynamics of the exposed loops are affected in the mutant such that these may impede the binding of the toxin.
Organizational Affiliation:
European Molecular Biology Laboratory, Heidelberg, Germany.