Insights into channel architecture and substrate specificity from crystal structures of two macrocycle-forming thioesterases of modular polyketide synthases
Tsai, S.-C., Lu, H., Cane, D.E., Khosla, C., Stroud, R.M.(2002) Biochemistry 41: 12598-12606
- PubMed: 12379102
- DOI: https://doi.org/10.1021/bi0260177
- Primary Citation of Related Structures:
1MN6, 1MNA, 1MNQ, 1MO2 - PubMed Abstract:
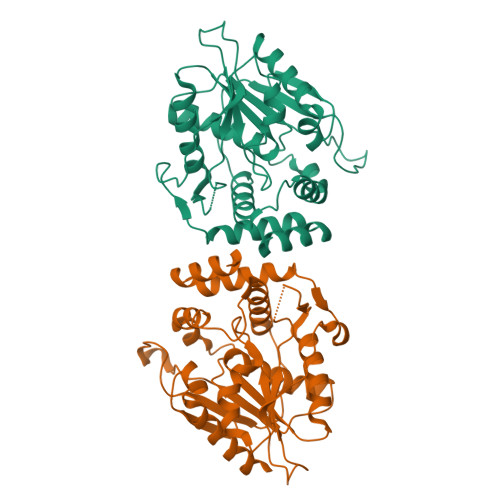
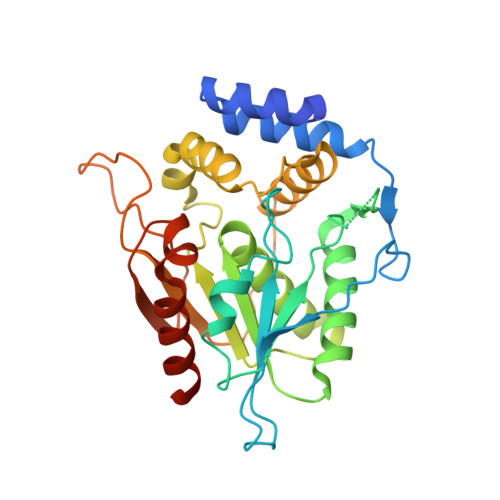
Modular polyketide synthases (PKSs) synthesize the polyketide cores of pharmacologically important natural products such as erythromycin and picromycin. Understanding PKSs at high resolution could present new opportunities for chemoenzymatic synthesis of complex molecules. The crystal structures of macrocycle-forming thioesterase (TE) domains from the picromycin synthase (PICS) and 6-deoxyerythronolide B synthase (DEBS) were determined to 1.8-3.0 A with an R(crys) of 19.2-24.4%, including three structures of PICS TE (crystallized at pH 7.6, 8.0, and 8.4) and a second crystal form of DEBS TE. As predicted by the previous work on DEBS TE [Tsai, S. C., et al. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 14808-14813], PICS TE contains an open substrate channel and a hydrophobic dimer interface. Notwithstanding their similarity, the dimer interfaces and substrate channels of DEBS TE and PICS TE reveal key differences. The structural basis for the divergent substrate specificities of DEBS TE and PICS TE is analyzed. The size of the substrate channel increases with increasing pH, presumably due to electrostatic repulsion in the channel at elevated pH. Together, these structures support previous predictions that macrocycle-forming thioesterases from PKSs share the same protein fold, an open substrate channel, a similar catalytic mechanism, and a hydrophobic dimer interface. They also provide a basis for the design of enzymes capable of catalyzing regioselective macrocyclization of natural or synthetic substrates. A series of high-resolution snapshots of a protein channel at different pHs is presented alongside analysis of channel residues, which could help in the redesign of the protein channel architecture.
Organizational Affiliation:
Department of Chemical Engineering, Stanford University, Stanford, California 94305-5025, USA.