Matrilysin-inhibitor complexes: common themes among metalloproteases.
Browner, M.F., Smith, W.W., Castelhano, A.L.(1995) Biochemistry 34: 6602-6610
- PubMed: 7756291
- DOI: https://doi.org/10.1021/bi00020a004
- Primary Citation of Related Structures:
1MMP, 1MMQ, 1MMR - PubMed Abstract:
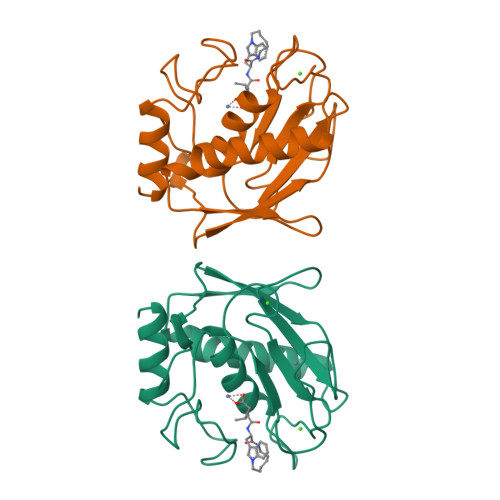
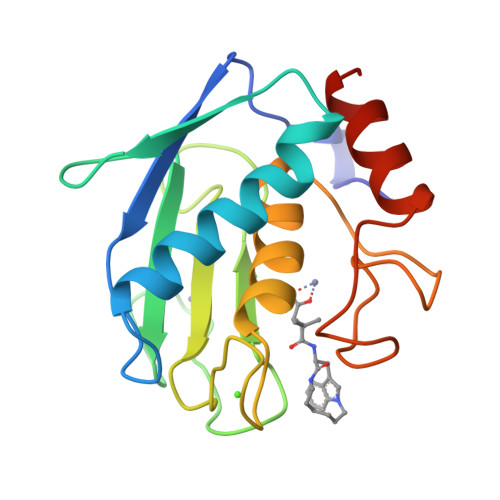
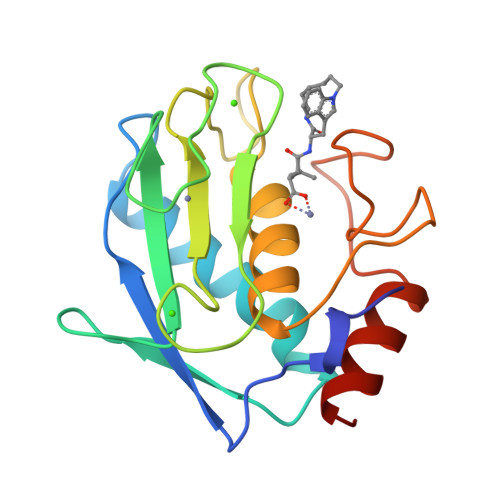
Matrix metalloproteases are a family of enzymes that play critical roles in the physiological and pathological degradation of the extracellular matrix. These enzymes may be important therapeutic targets for the treatment of various diseases where tissue degradation is part of the pathology, such as cancer and arthritis. Matrilysin is the smallest member of this family of enzymes, all of which require zinc for catalytic activity. The first X-ray crystal structures of human matrilysin are presented. Inhibitors of metalloproteases are often characterized by the chemical group that interacts with the active site zinc of the protein. The structures of matrilysin complexed with hydroxamate (maximum resolution 1.9 A), carboxylate (maximum resolution 2.4 A), and sulfodiimine (maximum resolution 2.3 A) inhibitors are presented here and provide detailed information about how each functional group interacts with the catalytic zinc. Only the zinc-coordination group is variable in this series of inhibitors. Examination of these inhibitor-matrilysin complexes emphasizes the dominant role the zinc-coordinating group plays in determining the relative potencies of the inhibitors. The structures of these matrilysin-inhibitor complexes also provide a basis for comparing the catalytic mechanism of MMPs and other metalloproteins.
Organizational Affiliation:
Molecular Structure Department, Syntex Discovery Research, Palo Alto, California 94303, USA.