Structural basis for the recognition of ldb1 by the N-terminal LIM domains of LMO2 and LMO4
Deane, J.E., Mackay, J.P., Kwan, A.H., Sum, E.Y., Visvader, J.E., Matthews, J.M.(2003) EMBO J 22: 2224-2233
- PubMed: 12727888
- DOI: https://doi.org/10.1093/emboj/cdg196
- Primary Citation of Related Structures:
1J2O, 1M3V - PubMed Abstract:
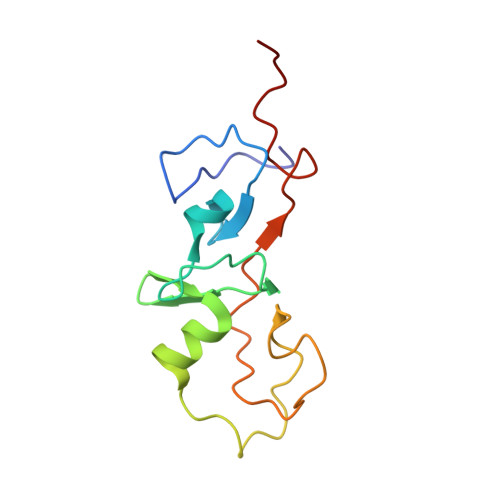
LMO2 and LMO4 are members of a small family of nuclear transcriptional regulators that are important for both normal development and disease processes. LMO2 is essential for hemopoiesis and angiogenesis, and inappropriate overexpression of this protein leads to T-cell leukemias. LMO4 is developmentally regulated in the mammary gland and has been implicated in breast oncogenesis. Both proteins comprise two tandemly repeated LIM domains. LMO2 and LMO4 interact with the ubiquitous nuclear adaptor protein ldb1/NLI/CLIM2, which associates with the LIM domains of LMO and LIM homeodomain proteins via its LIM interaction domain (ldb1-LID). We report the solution structures of two LMO:ldb1 complexes (PDB: 1M3V and 1J2O) and show that ldb1-LID binds to the N-terminal LIM domain (LIM1) of LMO2 and LMO4 in an extended conformation, contributing a third strand to a beta-hairpin in LIM1 domains. These findings constitute the first molecular definition of LIM-mediated protein-protein interactions and suggest a mechanism by which ldb1 can bind a variety of LIM domains that share low sequence homology.
- School of Molecular and Microbial Biosciences, University of Sydney, NSW 2006, Australia.
Organizational Affiliation: