Structural basis for the substrate specificity of tobacco etch virus protease.
Phan, J., Zdanov, A., Evdokimov, A.G., Tropea, J.E., Peters III, H.K., Kapust, R.B., Li, M., Wlodawer, A., Waugh, D.S.(2002) J Biological Chem 277: 50564-50572
- PubMed: 12377789
- DOI: https://doi.org/10.1074/jbc.M207224200
- Primary Citation of Related Structures:
1LVB, 1LVM - PubMed Abstract:
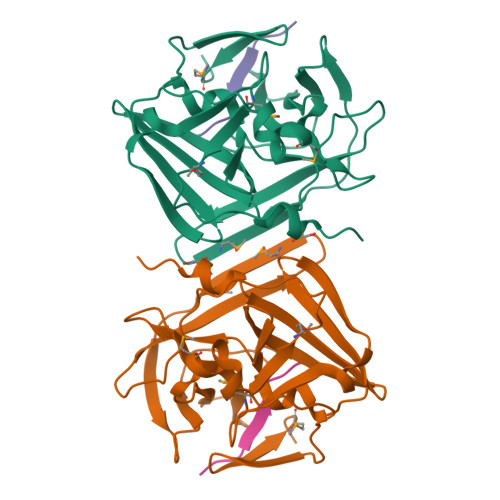
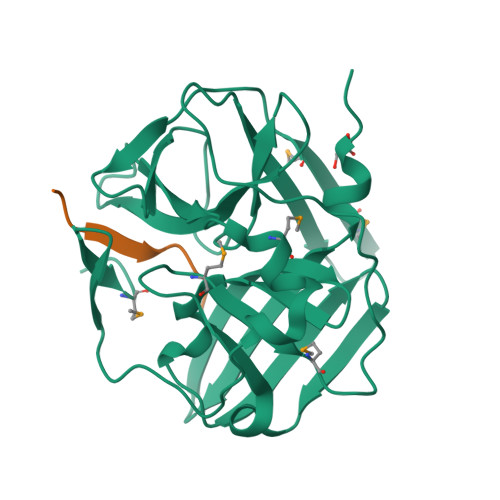
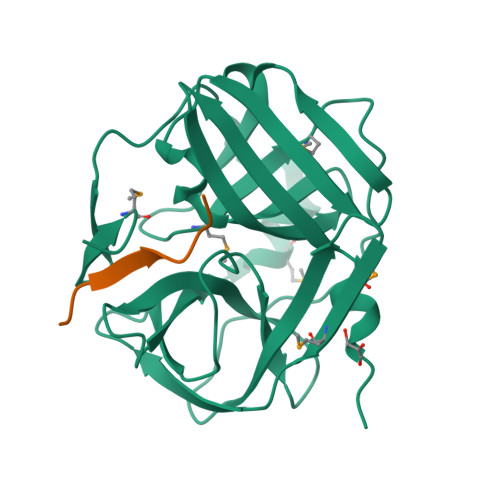
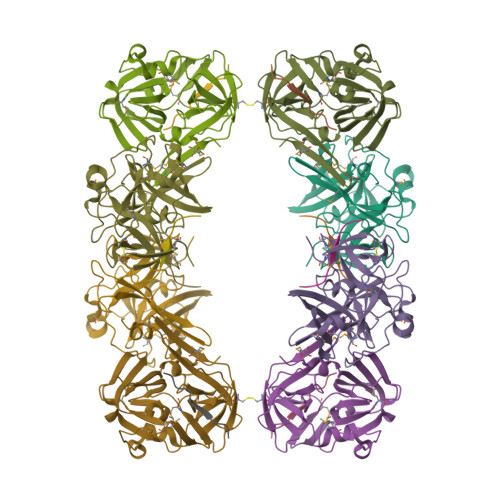
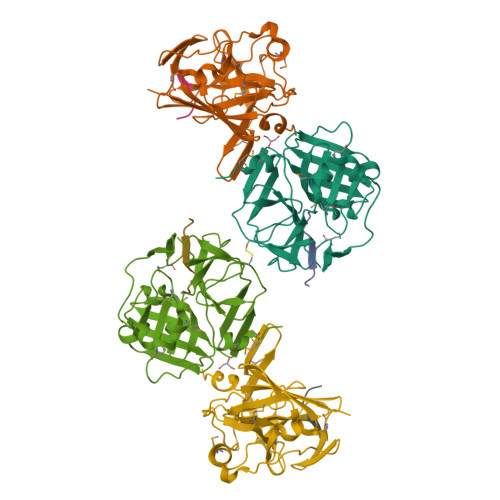
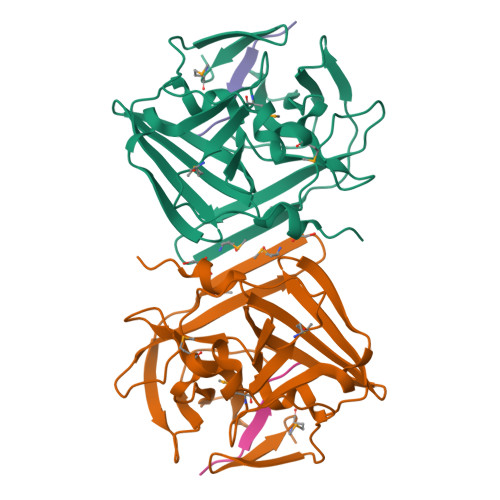
Because of its stringent sequence specificity, the 3C-type protease from tobacco etch virus (TEV) is frequently used to remove affinity tags from recombinant proteins. It is unclear, however, exactly how TEV protease recognizes its substrates with such high selectivity. The crystal structures of two TEV protease mutants, inactive C151A and autolysis-resistant S219D, have now been solved at 2.2- and 1.8-A resolution as complexes with a substrate and product peptide, respectively. The enzyme does not appear to have been perturbed by the mutations in either structure, and the modes of binding of the product and substrate are virtually identical. Analysis of the protein-ligand interactions helps to delineate the structural determinants of substrate specificity and provides guidance for reengineering the enzyme to further improve its utility for biotechnological applications.
Organizational Affiliation:
Macromolecular Crystallography Laboratory, Center for Cancer Research, NCI-Frederick, National Institutes of Health, Frederick, Maryland 21702-1201, USA.