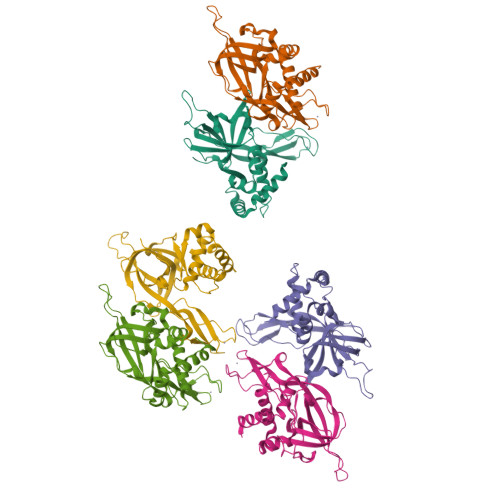
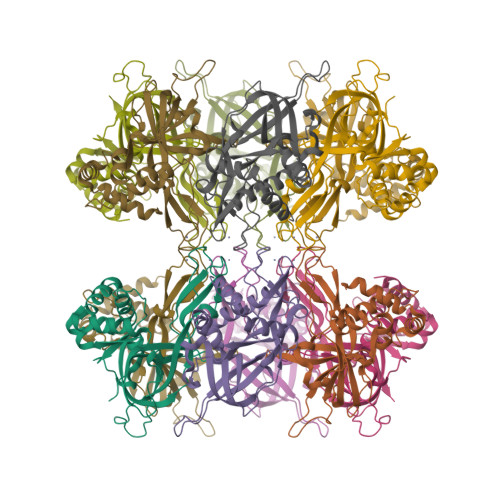
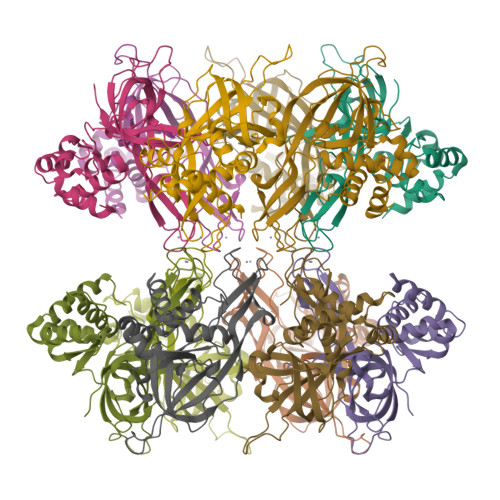
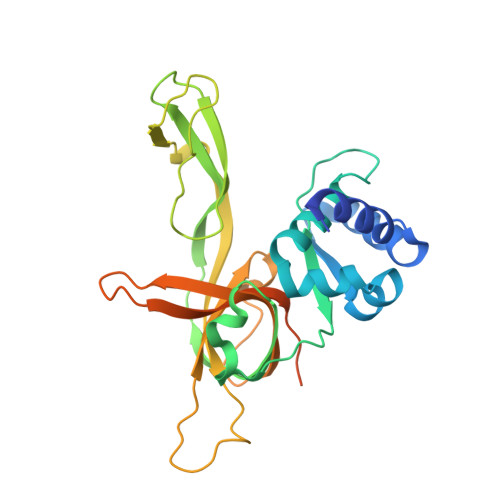
The Structure and function of MCM from archaeal M. Thermoautotrophicum
Fletcher, R.J., Bishop, B.E., Leon, R.P., Sclafani, R.A., Ogata, C.M., Chen, X.S.(2003) Nat Struct Biol 10: 160-167
- PubMed: 12548282
- DOI: https://doi.org/10.1038/nsb893
- Primary Citation of Related Structures:
1LTL - PubMed Abstract:
Eukaryotic chromosomal DNA is licensed for replication precisely once in each cell cycle. The mini-chromosome maintenance (MCM) complex plays a role in this replication licensing. We have determined the structure of a fragment of MCM from Methanobacterium thermoautotrophicum (mtMCM), a model system for eukaryotic MCM. The structure reveals a novel dodecameric architecture with a remarkably long central channel. The channel surface has an unusually high positive charge and binds DNA. We also show that the structure of the N-terminal fragment is conserved for all MCMs proteins despite highly divergent sequences, suggesting a common architecture for a similar task: gripping/remodeling DNA and regulating MCM activity. An mtMCM mutant protein equivalent to a yeast MCM5 (CDC46) protein with the bob1 mutation at its N terminus has only subtle structural changes, suggesting a Cdc7-bypass mechanism by Bob1 in yeast. Yeast bypass experiments using MCM5 mutant proteins support the hypothesis for the bypass mechanism.
Organizational Affiliation:
Department of Biochemistry and Molecular Genetics, University of Colorado Health Science Center, School of Medicine, Denver, Colorado 80262, USA.