DETECTION AND CHARACTERIZATION OF XENON-BINDING SITES IN PROTEINS BY 129XE NMR SPECTROSCOPY
Rubin, S.M., Lee, S.-Y., Ruiz, E.J., Pines, A., Wemmer, D.E.(2002) J Mol Biology 322: 425-440
- PubMed: 12217701
- DOI: https://doi.org/10.1016/s0022-2836(02)00739-8
- Primary Citation of Related Structures:
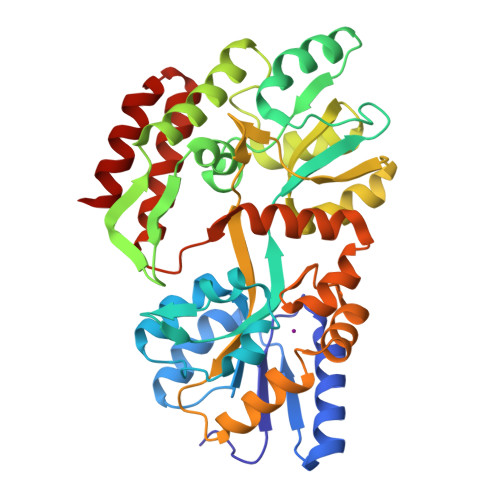
1LLS - PubMed Abstract:
Xenon-binding sites in proteins have led to a number of applications of xenon in biochemical and structural studies. Here we further develop the utility of 129Xe NMR in characterizing specific xenon-protein interactions. The sensitivity of the 129Xe chemical shift to its local environment and the intense signals attainable by optical pumping make xenon a useful NMR reporter of its own interactions with proteins. A method for detecting specific xenon-binding interactions by analysis of 129Xe chemical shift data is illustrated using the maltose binding protein (MBP) from Escherichia coli as an example. The crystal structure of MBP in the presence of 8atm of xenon confirms the binding site determined from NMR data. Changes in the structure of the xenon-binding cavity upon the binding of maltose by the protein can account for the sensitivity of the 129Xe chemical shift to MBP conformation. 129Xe NMR data for xenon in solution with a number of cavity containing phage T4 lysozyme mutants show that xenon can report on cavity structure. In particular, a correlation exists between cavity size and the binding-induced 129Xe chemical shift. Further applications of 129Xe NMR to biochemical assays, including the screening of proteins for xenon binding for crystallography are considered.
Organizational Affiliation:
Department of Chemistry, MC-1460, University of California, Berkeley 94720-1460, USA.