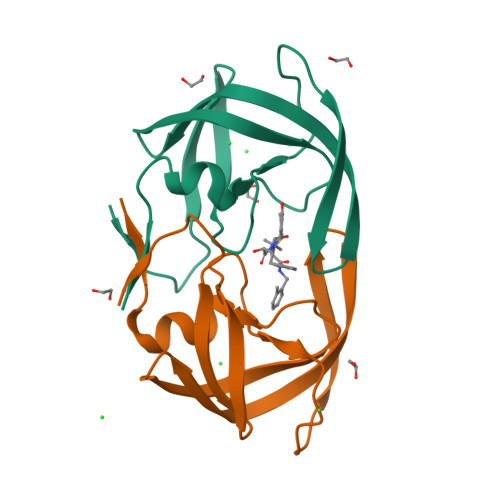
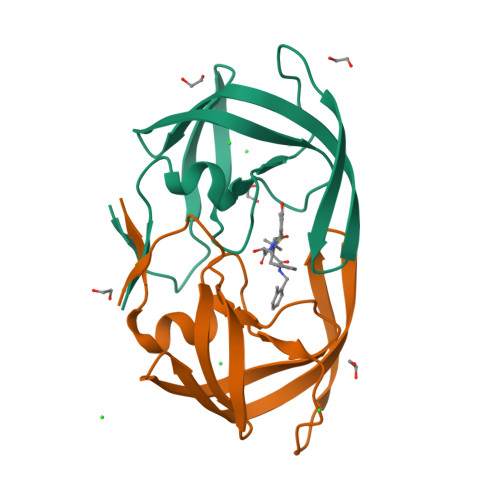
Anisotropic Dynamics of the JE-2147-HIV Protease Complex: Drug Resistance and Thermodynamic Binding Mode Examined in a 1.09 A Structure
Reiling, K.K., Endres, N.F., Dauber, D.S., Craik, C.S., Stroud, R.M.(2002) Biochemistry 41: 4582-4594
- PubMed: 11926820
- DOI: https://doi.org/10.1021/bi011781z
- Primary Citation of Related Structures:
1KZK - PubMed Abstract:
The structure of HIV protease (HIV Pr) bound to JE-2147 (also named AG1776 or KNI-764) is determined here to 1.09 A resolution. This highest-resolution structure for HIV Pr allows refinement of anisotropic displacement parameters (ADPs) for all atoms. Clustering based on the directional information in ADPs defines two sets of subdomains such that within each set, subdomains undergo similar anisotropic motion. These sets are (a) the core of monomer A grouped with both substrate-binding flaps and (b) the core of monomer B coupled to both catalytic aspartates (25A/B). The four-stranded beta-sheet (1-4 A/B and 95-99 A/B) that forms a significant part of the dimer interface exhibits large anisotropic amplitudes that differ from those of the other sets of subdomains. JE-2147 is shown here to be a picomolar inhibitor (K(i) = 41 +/- 18 pM). The structure is used to interpret the mechanism of association of JE-2147, a second-generation inhibitor for which binding is enthalpically driven, with respect to first-generation inhibitors for which binding is predominantly entropically driven [Velazquez-Campoy, A., et al. (2001) Arch. Biochem. Biophys. 390, 169-175]. Relative to the entropically driven inhibitor complexes, the JE-2147-HIV Pr complex exhibits an approximately 0.5 A movement of the substrate flaps in toward the substrate, suggesting a more compatible enthalpically driven association. Domains of the protease identified by clustering of ADPs also suggest a model of enthalpy-entropy compensation for all HIV Pr inhibitors in which dynamic coupling of the flaps is offset by an increased level of motion of the beta-sheet domain of the dimer interface (1-4 A/B and 95-99 A/B).
Organizational Affiliation:
Department of Biochemistry and Biophysics, Graduate Group in Biophysics, University of California at San Francisco, San Francisco, California 94143, USA.