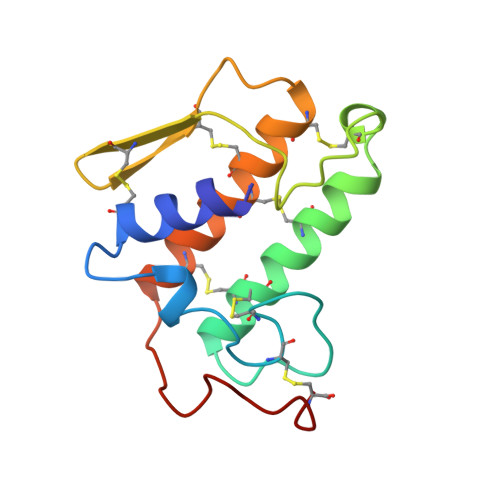
Structures of the catalytic site mutants D99A and H48Q and the calcium-loop mutant D49E of phospholipase A2.
Sekar, K., Biswas, R., Li, Y., Tsai, M., Sundaralingam, M.(1999) Acta Crystallogr D Biol Crystallogr 55: 443-447
- PubMed: 10089353
- DOI: https://doi.org/10.1107/s0907444998013699
- Primary Citation of Related Structures:
1KVW, 1KVX, 1KVY - PubMed Abstract:
Crystal structures of the active-site mutants D99A and H48Q and the calcium-loop mutant D49E of bovine phospholipase A2 have been determined at around 1.9 A resolution. The D99A mutant is isomorphous to the orthorhombic recombinant enzyme, space group P212121. The H48Q and the calcium-loop mutant D49E are isomorphous to the trigonal recombinant enzyme, space group P3121. The two active-site mutants show no major structural perturbations. The structural water is absent in D99A and, therefore, the hydrogen-bonding scheme is changed. In H48Q, the catalytic water is present and hydrogen bonded to Gln48 N, but the second water found in native His48 is absent. In the calcium-loop mutant D49E, the two water molecules forming the pentagonal bipyramid around calcium are absent and only one O atom of the Glu49 carboxylate group is coordinated to calcium, resulting in only four ligands.
Organizational Affiliation:
Biological Macromolecular Structure Center, Department of Chemistry, Ohio State Biochemistry Program, 012 Rightmire Hall, 1060 Carmack Road, The Ohio State University, Columbus, OH 43210, USA.