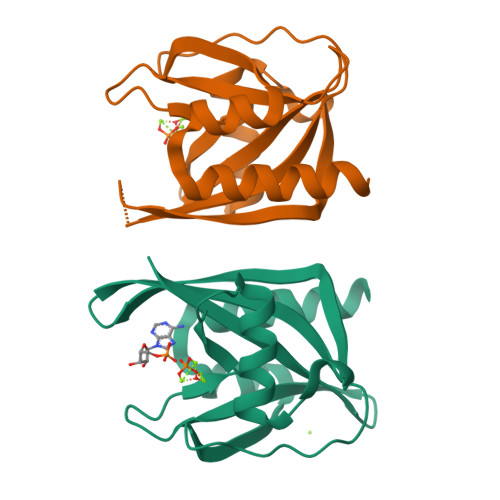
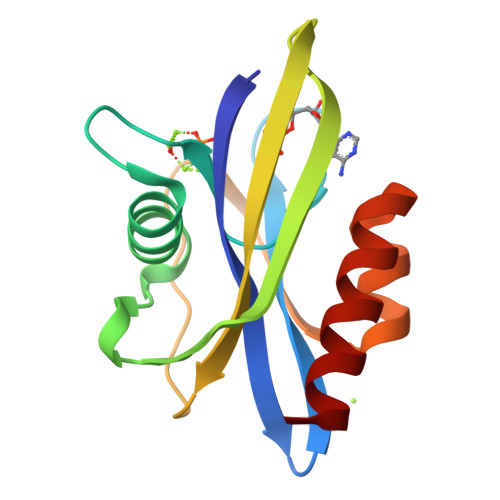
The crystal structure of diadenosine tetraphosphate hydrolase from Caenorhabditis elegans in free and binary complex forms
Bailey, S., Sedelnikova, S.E., Blackburn, G.M., Abdelghany, H.M., Baker, P.J., McLennan, A.G., Rafferty, J.B.(2002) Structure 10: 589-600
- PubMed: 11937063
- DOI: https://doi.org/10.1016/s0969-2126(02)00746-3
- Primary Citation of Related Structures:
1KT9, 1KTG - PubMed Abstract:
The crystal structure of C. elegans Ap(4)A hydrolase has been determined for the free enzyme and a binary complex at 2.0 A and 1.8 A, respectively. Ap(4)A hydrolase has a key role in regulating the intracellular Ap(4)A levels and hence potentially the cellular response to metabolic stress and/or differentiation and apoptosis via the Ap(3)A/Ap(4)A ratio. The structures reveal that the enzyme has the mixed alpha/beta fold of the Nudix family and also show how the enzyme binds and locates its substrate with respect to the catalytic machinery of the Nudix motif. These results suggest how the enzyme can catalyze the hydrolysis of a range of related dinucleoside tetraphosphate, but not triphosphate, compounds through precise orientation of key elements of the substrate.
Organizational Affiliation:
Krebs Institute for Biomolecular Research, Department of Molecular Biology and Biotechnology, University of Sheffield, Western Bank, United Kingdom.