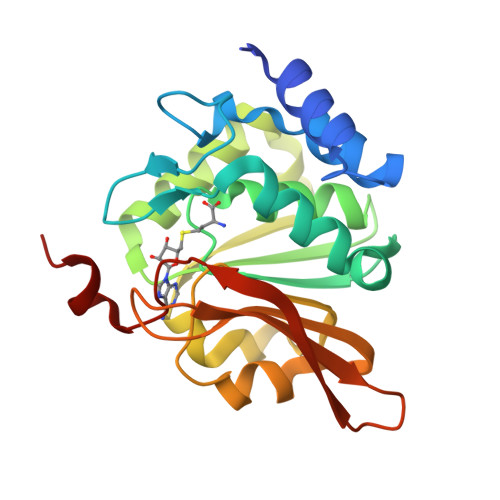
Crystal structure of human L-isoaspartyl methyltransferase.
Ryttersgaard, C., Griffith, S.C., Sawaya, M.R., MacLaren, D.C., Clarke, S., Yeates, T.O.(2002) J Biological Chem 277: 10642-10646
- PubMed: 11792715
- DOI: https://doi.org/10.1074/jbc.M200229200
- Primary Citation of Related Structures:
1KR5 - PubMed Abstract:
The enzyme l-isoaspartyl methyltransferase initiates the repair of damaged proteins by recognizing and methylating isomerized and racemized aspartyl residues in aging proteins. The crystal structure of the human enzyme containing a bound S-adenosyl-l-homocysteine cofactor is reported here at a resolution of 2.1 A. A comparison of the human enzyme to homologs from two other species reveals several significant differences among otherwise similar structures. In all three structures, we find that three conserved charged residues are buried in the protein interior near the active site. Electrostatics calculations suggest that these buried charges might make significant contributions to the energetics of binding the charged S-adenosyl-l-methionine cofactor and to catalysis. We suggest a possible structural explanation for the observed differences in reactivity toward the structurally similar l-isoaspartyl and d-aspartyl residues in the human, archael, and eubacterial enzymes. Finally, the human structure reveals that the known genetic polymorphism at residue 119 (Val/Ile) maps to an exposed region away from the active site.
Organizational Affiliation:
Department of Chemistry and Biochemistry, University of California, Los Angeles, California 90095-1569, USA.