Giant protein kinases: domain interactions and structural basis of autoregulation.
Kobe, B., Heierhorst, J., Feil, S.C., Parker, M.W., Benian, G.M., Weiss, K.R., Kemp, B.E.(1996) EMBO J 15: 6810-6821
- PubMed: 9003756
- Primary Citation of Related Structures:
1KOA, 1KOB - PubMed Abstract:
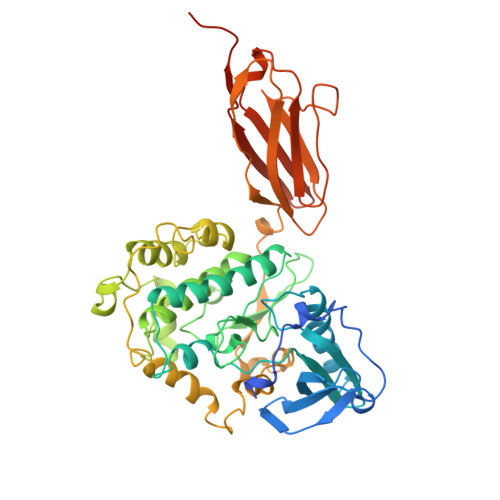
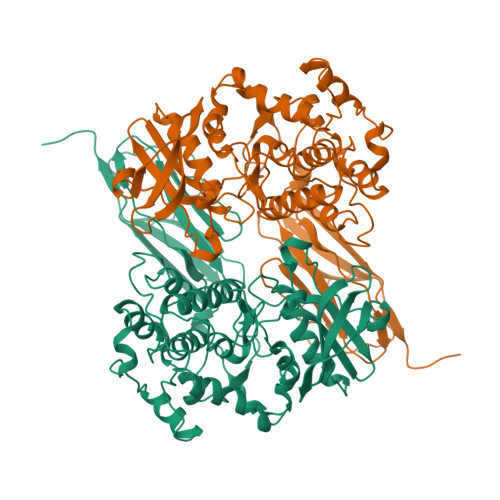
The myosin-associated giant protein kinases twitchin and titin are composed predominantly of fibronectin- and immunoglobulin-like modules. We report the crystal structures of two autoinhibited twitchin kinase fragments, one from Aplysia and a larger fragment from Caenorhabditis elegans containing an additional C-terminal immunoglobulin-like domain. The structure of the longer fragment shows that the immunoglobulin domain contacts the protein kinase domain on the opposite side from the catalytic cleft, laterally exposing potential myosin binding residues. Together, the structures reveal the cooperative interactions between the autoregulatory region and the residues from the catalytic domain involved in protein substrate binding, ATP binding, catalysis and the activation loop, and explain the differences between the observed autoinhibitory mechanism and the one found in the structure of calmodulin-dependent kinase I.
Organizational Affiliation:
St. Vincent's Institute of Medical Research, Fitzroy, Victoria, Australia.