Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR.
Feinberg, H., Mitchell, D.A., Drickamer, K., Weis, W.I.(2001) Science 294: 2163-2166
- PubMed: 11739956
- DOI: https://doi.org/10.1126/science.1066371
- Primary Citation of Related Structures:
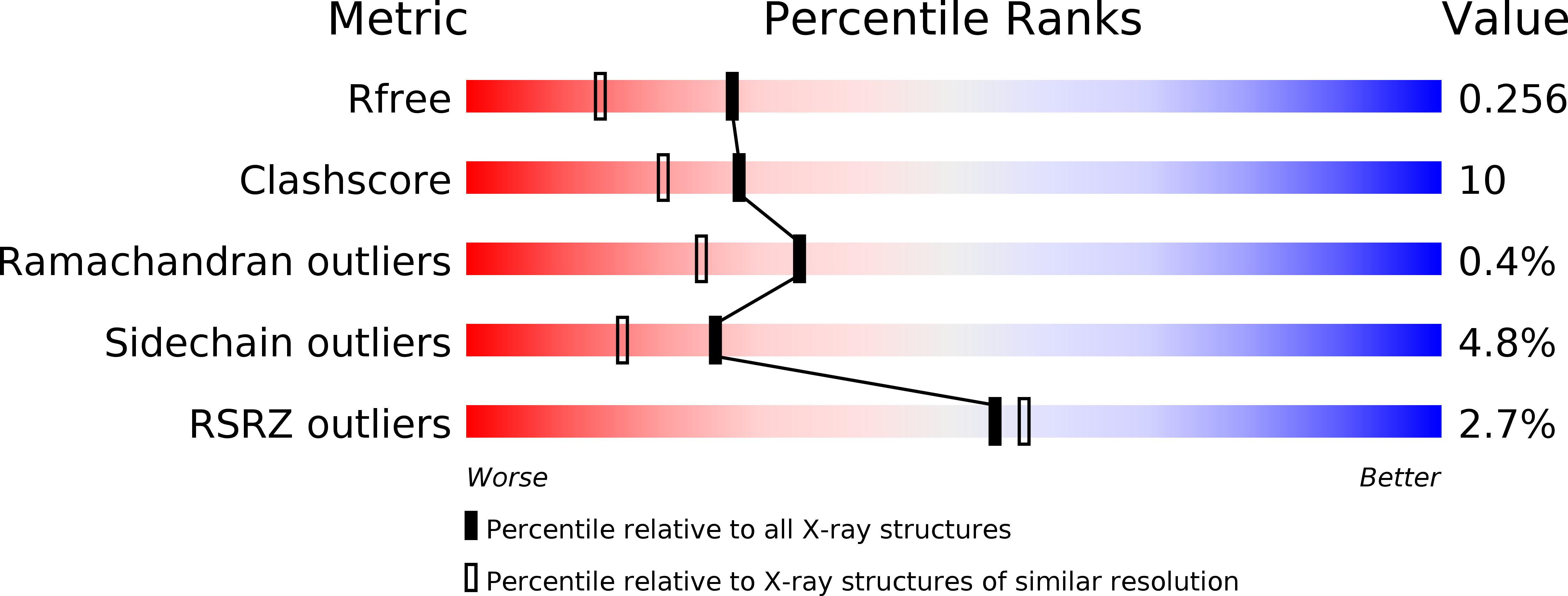
1K9I, 1K9J - PubMed Abstract:
Dendritic cell specific intracellular adhesion molecule-3 (ICAM-3) grabbing nonintegrin (DC-SIGN), a C-type lectin present on the surface of dendritic cells, mediates the initial interaction of dendritic cells with T cells by binding to ICAM-3. DC-SIGN and DC-SIGNR, a related receptor found on the endothelium of liver sinusoids, placental capillaries, and lymph nodes, bind to oligosaccharides that are present on the envelope of human immunodeficiency virus (HIV), an interaction that strongly promotes viral infection of T cells. Crystal structures of carbohydrate-recognition domains of DC-SIGN and of DC-SIGNR bound to oligosaccharide, in combination with binding studies, reveal that these receptors selectively recognize endogenous high-mannose oligosaccharides and may represent a new avenue for developing HIV prophylactics.
Organizational Affiliation:
Department of Structural Biology, University School of Medicine, Stanford, CA 94305, USA.