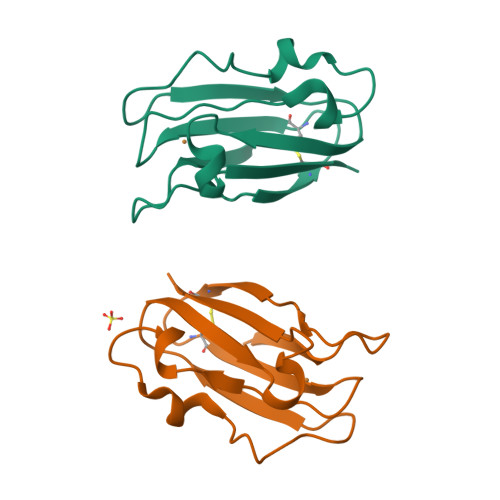
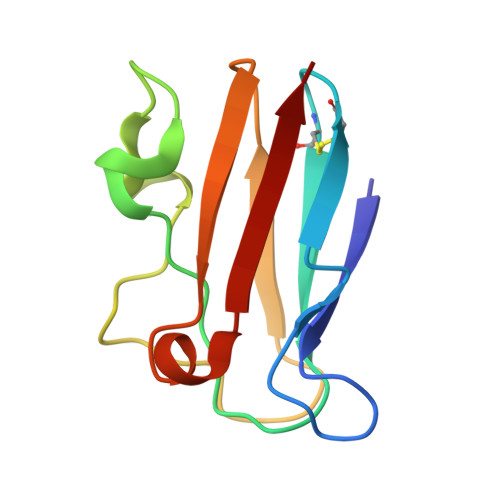
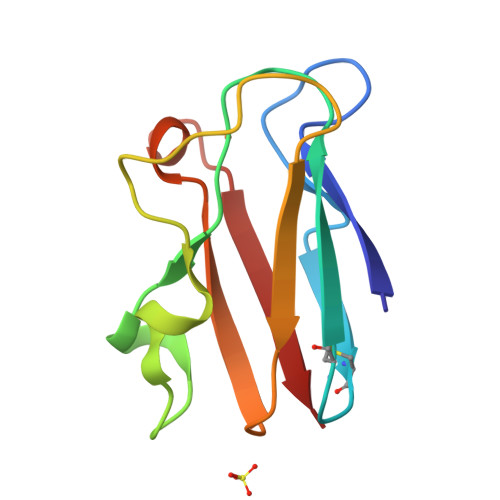
The 1.6 A resolution crystal structure of a mutant plastocyanin bearing a 21-25 engineered disulfide bridge.
Milani, M., Andolfi, L., Cannistraro, S., Verbeet, M.P., Bolognesi, M.(2001) Acta Crystallogr D Biol Crystallogr 57: 1735-1738
- PubMed: 11679761
- DOI: https://doi.org/10.1107/s0907444901013221
- Primary Citation of Related Structures:
1JXG - PubMed Abstract:
Plastocyanin is an electron-transfer protein which has been largely used for biophysical studies as well as for protein-engineering experiments. A surface disulfide bridge has been engineered in poplar plastocyanin to allow protein chemisorption on gold substrates. The mutated plastocyanin crystal structure has been studied at 1.6 A resolution (R factor = 0.145, R(free) = 0.205) to characterize the effects of the engineered disulfide on the overall protein structure and on the Cu-coordination sphere in view of biophysical applications. The new orthorhombic crystal form isolated for the mutated plastocyanin displays two protein molecules per asymmetric unit.
Organizational Affiliation:
Department of Physics-INFM, c/o Advanced Biotechnology Center-IST, University of Genova, Largo Rosanna Benzi 10, 16132 Genova, Italy.