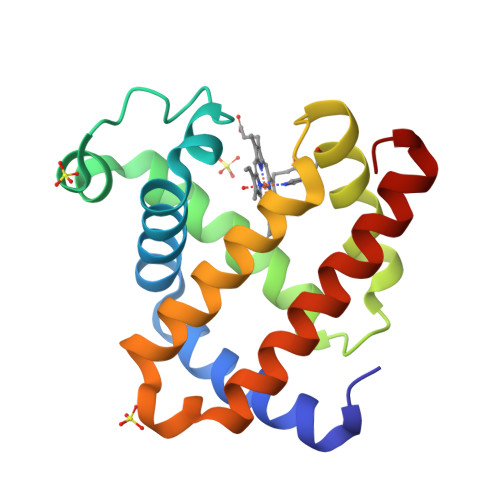
Probing substates in sperm whale myoglobin using high-pressure crystallography.
Urayama, P., Phillips Jr., G.N., Gruner, S.M.(2002) Structure 10: 51-60
- PubMed: 11796110
- DOI: https://doi.org/10.1016/s0969-2126(01)00699-2
- Primary Citation of Related Structures:
1JP6, 1JP8, 1JP9, 1JPB - PubMed Abstract:
Pressures in the 100 MPa range are known to have an enormous number of effects on the action of proteins, but straightforward means for determining the structural basis of these effects have been lacking. Here, crystallography has been used to probe effects of pressure on sperm whale myoglobin structure. A comparison of pressure effects with those seen at low pH suggests that structural changes under pressure are interpretable as a shift in the populations of conformational substates. Furthermore, a novel high-pressure protein crystal-cooling method has been used to show low-temperature metastability, providing an alternative to room temperature, beryllium pressure cell-based techniques. The change in protein structure due to pressure is not purely compressive and involves conformational changes important to protein activity. Correlation with low-pH structures suggests observed structural changes are associated with global conformational substates. Methods developed here open up a direct avenue for exploration of the effects of pressure on proteins.
Organizational Affiliation:
Department of Physics, Cornell University, Ithaca, NY 14853, USA.