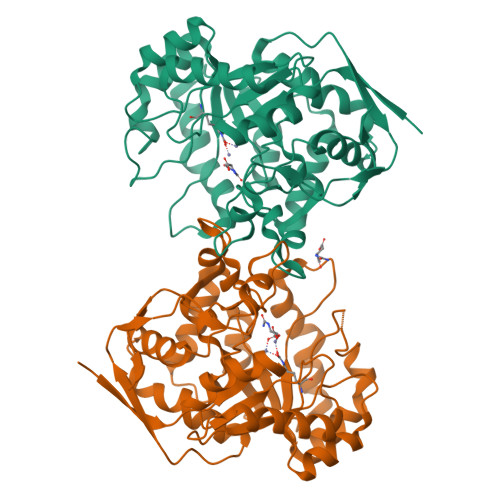
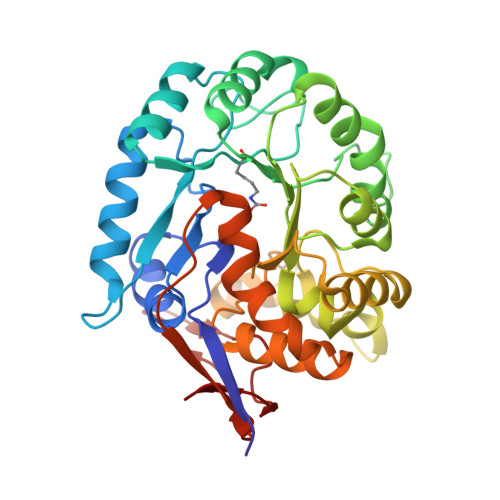
Molecular structure of dihydroorotase: a paradigm for catalysis through the use of a binuclear metal center.
Thoden, J.B., Phillips Jr., G.N., Neal, T.M., Raushel, F.M., Holden, H.M.(2001) Biochemistry 40: 6989-6997
- PubMed: 11401542
- DOI: https://doi.org/10.1021/bi010682i
- Primary Citation of Related Structures:
1J79 - PubMed Abstract:
Dihydroorotase plays a key role in pyrimidine biosynthesis by catalyzing the reversible interconversion of carbamoyl aspartate to dihydroorotate. Here we describe the three-dimensional structure of dihydroorotase from Escherichia coli determined and refined to 1.7 A resolution. Each subunit of the homodimeric enzyme folds into a "TIM" barrel motif with eight strands of parallel beta-sheet flanked on the outer surface by alpha-helices. Unexpectedly, each subunit contains a binuclear zinc center with the metal ions separated by approximately 3.6 A. Lys 102, which is carboxylated, serves as a bridging ligand between the two cations. The more buried or alpha-metal ion in subunit I is surrounded by His 16, His 18, Lys 102, Asp 250, and a solvent molecule (most likely a hydroxide ion) in a trigonal bipyramidal arrangement. The beta-metal ion, which is closer to the solvent, is tetrahedrally ligated by Lys 102, His 139, His 177, and the bridging hydroxide. L-Dihydroorotate is observed bound to subunit I, with its carbonyl oxygen, O4, lying 2.9 A from the beta-metal ion. Important interactions for positioning dihydroorotate into the active site include a salt bridge with the guanidinium group of Arg 20 and various additional electrostatic interactions with both protein backbone and side chain atoms. Strikingly, in subunit II, carbamoyl L-aspartate is observed binding near the binuclear metal center with its carboxylate side chain ligating the two metals and thus displacing the bridging hydroxide ion. From the three-dimensional structures of the enzyme-bound substrate and product, it has been possible to propose a unique catalytic mechanism for dihydroorotase. In the direction of dihydroorotate hydrolysis, the bridging hydroxide attacks the re-face of dihydroorotate with general base assistance by Asp 250. The carbonyl group is polarized for nucleophilic attack by the bridging hydroxide through a direct interaction with the beta-metal ion. During the cyclization of carbamoyl aspartate, Asp 250 initiates the reaction by abstracting a proton from N3 of the substrate. The side chain carboxylate of carbamoyl aspartate is polarized through a direct electrostatic interaction with the binuclear metal center. The ensuing tetrahedral intermediate collapses with C-O bond cleavage and expulsion of the hydroxide which then bridges the binuclear metal center.
Organizational Affiliation:
Department of Biochemistry, University of Wisconsin, Madison, Wisconsin 53706, USA.