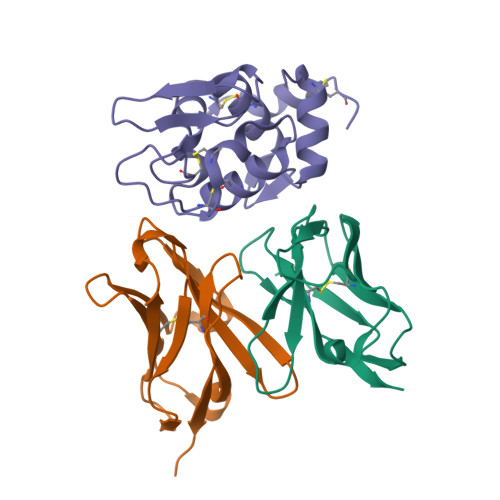
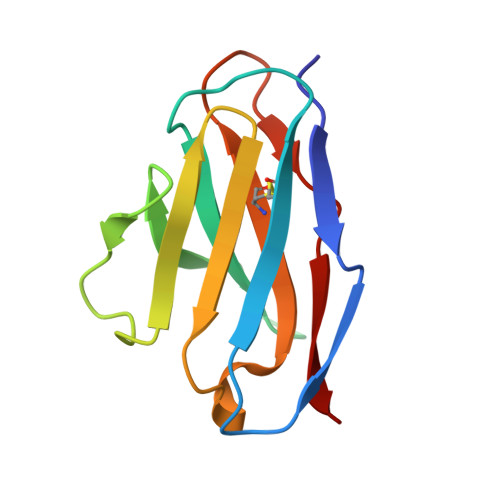
The Role of Hydrogen Bonding via Interfacial Water Molecules in Antigen-Antibody Complexation. THE HyHEL-10-HEL INTERACTION
Yokota, A., Tsumoto, K., Shiroishi, M., Kondo, H., Kumagai, I.(2003) J Biological Chem 278: 5410-5418
- PubMed: 12444085
- DOI: https://doi.org/10.1074/jbc.M210182200
- Primary Citation of Related Structures:
1J1O, 1J1P, 1J1X - PubMed Abstract:
To study the role of hydrogen bonding via interfacial water molecules in protein-protein interactions, we examined the interaction between hen egg white lysozyme (HEL) and its HyHEL-10 variable domain fragment (Fv) antibody. We constructed three antibody mutants (l-Y50F, l-S91A, and l-S93A) and investigated the interactions between the mutant Fvs and HEL. Isothermal titration calorimetry indicated that the mutations significantly decreased the negative enthalpy change (8-25 kJ mol(-1)), despite some offset by a favorable entropy change. X-ray crystallography demonstrated that the complexes had nearly identical structures, including the positions of the interfacial water molecules. Taken together, the isothermal titration calorimetric and x-ray crystallographic results indicate that hydrogen bonding via interfacial water enthalpically contributes to the Fv-HEL interaction despite the partial offset because of entropy loss, suggesting that hydrogen bonding stiffens the antigen-antibody complex.
Organizational Affiliation:
Department of Biomolecular Engineering, Graduate School of Engineering, Tohoku University, Aoba-yama 07, Aoba-ku, Sendai 980-8579, Japan.