Crystal structure of a histidine-tagged serine hydrolase involved in the carbazole degradation (CarC enzyme).
Habe, H., Morii, K., Fushinobu, S., Nam, J.W., Ayabe, Y., Yoshida, T., Wakagi, T., Yamane, H., Nojiri, H., Omori, T.(2003) Biochem Biophys Res Commun 303: 631-639
- PubMed: 12659866
- DOI: https://doi.org/10.1016/s0006-291x(03)00375-9
- Primary Citation of Related Structures:
1J1I - PubMed Abstract:
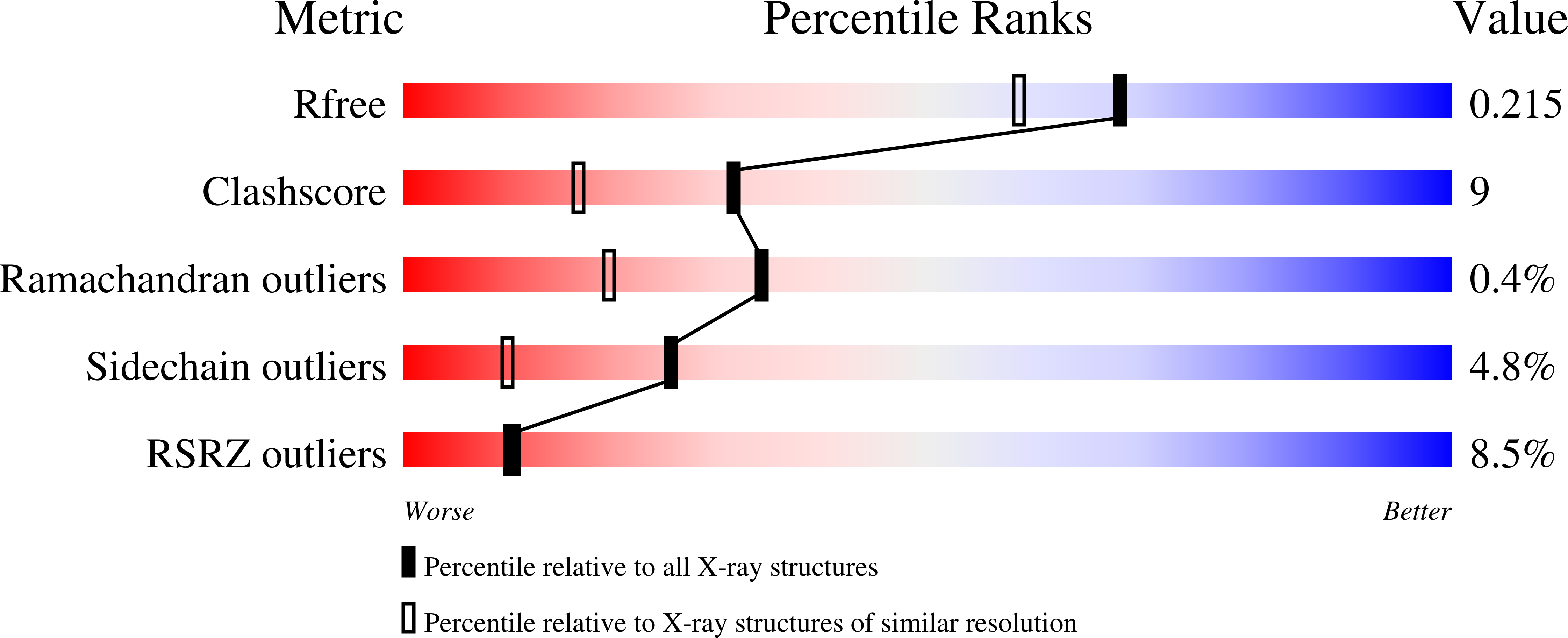
2-Hydroxy-6-oxo-6-(2(')-aminophenyl)-hexa-2,4-dienoate hydrolases (CarC enzymes) from two carbazole-degrading bacteria were purified using recombinant Escherichia coli strains with the histidine (His)-tagged purification system. The His-tagged CarC (ht-CarC) enzymes from Pseudomonas resinovorans strain CA10 (ht-CarC(CA10)) and Janthinobacterium sp. strain J3 (ht-CarC(J3)) exhibited hydrolase activity toward 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate as the purified native CarC(CA10) did. ht-CarC(J3) was crystallized in the space group I422 with cell dimensions of a=b=130.3A, c=84.5A in the hexagonal setting, and the crystal structure of ht-CarC(J3) was determined at 1.86A resolution. The final refined model of ht-CarC(J3) yields an R-factor of 21.6%, although the electron-density corresponding to Ile146 to Asn155 was ambiguous in the final model. We compared the known structures of BphD from Rhodococcus sp. strain RHA1 and CumD from Pseudomonas fluorescens strain IP01. The backbone conformation of ht-CarC(J3) was better superimposed with CumD than with BphD(RHA1). The side-chain directions of Arg185 and Trp262 residues in the substrate binding pockets of these enzymes were different among these proteins, suggesting that these residues may take a conformational change during the catalytic cycles.
Organizational Affiliation:
Biotechnology Research Center, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-8657, Japan.