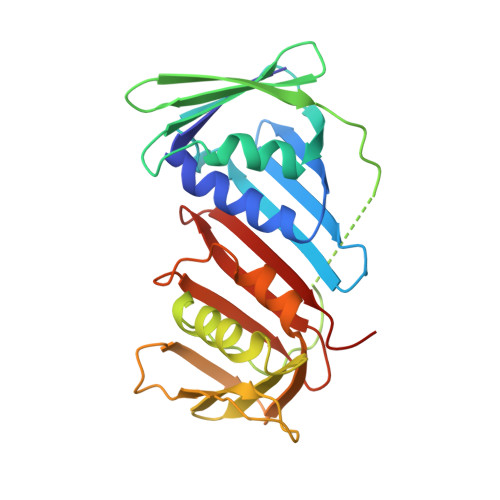
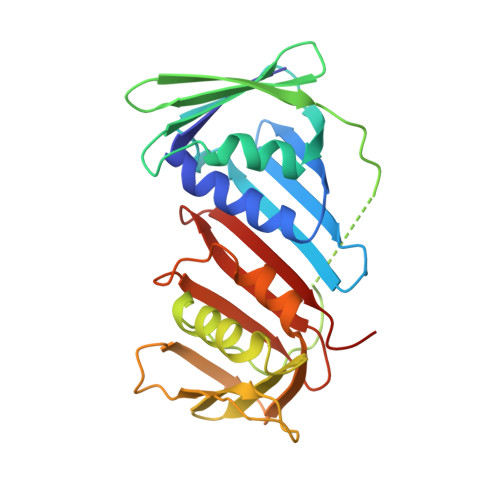
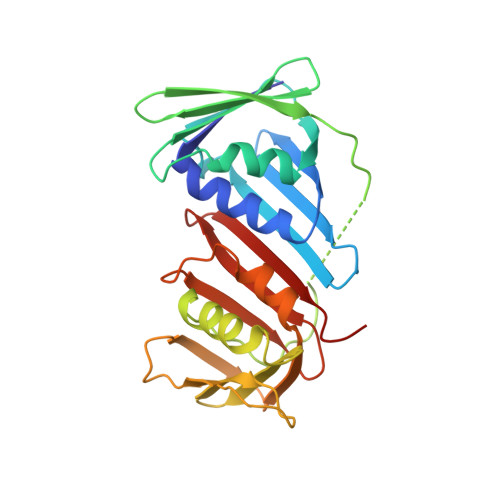
Intermolecular ion pairs maintain the toroidal structure of Pyrococcus furiosus PCNA
Matsumiya, S., Ishino, S., Ishino, Y., Morikawa, K.(2003) Protein Sci 12: 823-831
- PubMed: 12649440
- DOI: https://doi.org/10.1110/ps.0234503
- Primary Citation of Related Structures:
1IZ4, 1IZ5 - PubMed Abstract:
Two mutant proliferating cell nuclear antigens from the hyperthermophilic archaeon Pyrococcus furiosus, PfuPCNA(D143A) and PfuPCNA(D143A/D147A), were prepared by site-specific mutagenesis. The results from gel filtration showed that mutations at D143 and D147 drastically affect the stability of the trimeric structure of PfuPCNA. The PfuPCNA(D143A) still retained the activity to stimulate the DNA polymerase reaction, but PfuPCNA(D143A/D147A) lost the activity. Crystal structures of the mutant PfuPCNAs were determined. Although the wild-type PCNA forms a toroidal trimer with intermolecular hydrogen bonds between the N- and C-terminal domains, the mutant PfuPCNAs exist as V-shaped dimers through intermolecular hydrogen bonds between the two C-terminal domains in the crystal. Because the mutated residues are involved in the intermolecular ion pairs through their side chains in the wild-type PfuPCNA, these ion pairs seem to play a key role in maintaining the toroidal structure of the PfuPCNA trimer. The comparison of the crystal structures revealed intriguing conformational flexibility of each domain in the PfuPCNA subunit. This structural versatility of PCNA may be involved in the mechanisms for ring opening and closing.
Organizational Affiliation:
Department of Structural Biology, Biomolecular Engineering Research Institute, Osaka 565-0874, Japan.