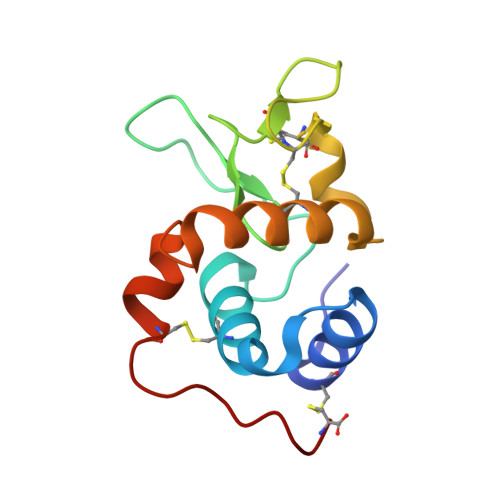
Structure of the induced antibacterial protein from tasar silkworm, Antheraea mylitta. Implications to molecular evolution.
Jain, D., Nair, D.T., Swaminathan, G.J., Abraham, E.G., Nagaraju, J., Salunke, D.M.(2001) J Biol Chem 276: 41377-41382
- PubMed: 11522783
- DOI: https://doi.org/10.1074/jbc.M104674200
- Primary Citation of Related Structures:
1IIZ - PubMed Abstract:
The crystal structure of an antibacterial protein of immune origin (TSWAB), purified from tasar silkworm (Antheraea mylitta) larvae after induction by Escherichia coli infection, has been determined. This is the first insect lysozyme structure and represents induced lysozymes of innate immunity. The core structure of TSWAB is similar to c-type lysozymes and alpha-lactalbumins. However, TSWAB shows significant differences with respect to the other two proteins in the exposed loop regions. The catalytic residues in TSWAB are conserved with respect to the chicken lysozyme, indicating a common mechanism of action. However, differences in the noncatalytic residues in the substrate binding groove imply subtle differences in the specificity and the level of activity. Thus, conformational differences between TSWAB and chicken lysozyme exist, whereas functional mechanisms appear to be similar. On the other hand, alpha-lactalbumins and c-type lysozymes exhibit drastically different functions with conserved molecular conformation. It is evident that a common molecular scaffold is exploited in the three enzymes for apparently different physiological roles. It can be inferred on the basis of the structure-function comparison of these three proteins having common phylogenetic origin that the conformational changes in a protein are minimal during rapid evolution as compared with those in the normal course of evolution.
Organizational Affiliation:
National Institute of Immunology, New Delhi 110 067, India.