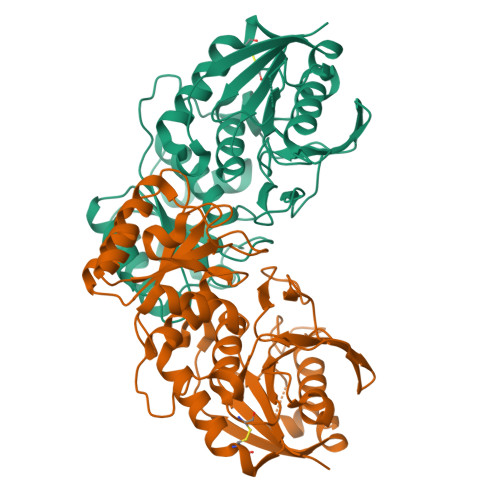
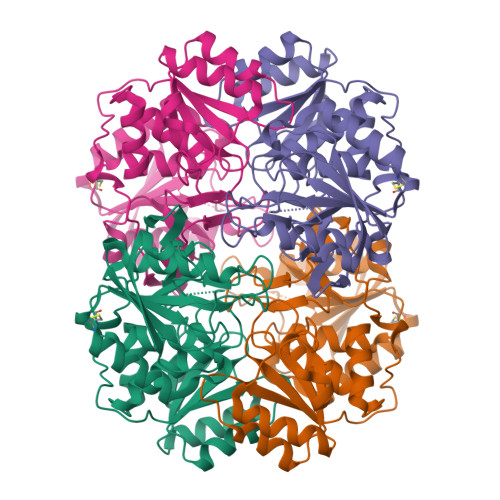
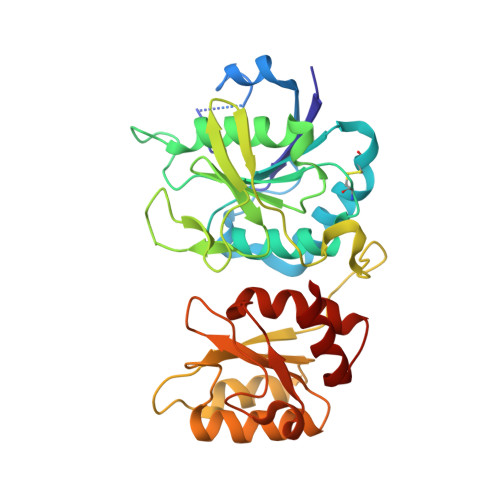
Crystal structure of active site mutant of antileukemic L-asparaginase reveals conserved zinc-binding site.
Borek, D., Kozak, M., Pei, J., Jaskolski, M.(2014) FEBS J 281: 4097-4111
- PubMed: 25040257
- DOI: https://doi.org/10.1111/febs.12906
- Primary Citation of Related Structures:
1IHD, 1JAZ, 1JJA - PubMed Abstract:
The periplasmic enzyme l-asparaginase type II from Escherichia coli (EcAII) converts l-asparagine to l-aspartate and ammonia. EcAII is an important drug in the treatment of childhood acute lymphoblastic leukemia, the most common malignancy in children. Leukemic blast cells lack the ability to synthesize l-asparagine and rely on other sources of l-asparagine for protein synthesis. EcAII injections deplete extracellular levels of l-asparagine, disrupting protein synthesis and inducing apoptosis in the malignant cells. The detailed mechanism of l-asparaginase catalytic action, the molecular mechanisms of its anticancer activity and the side effects associated with the treatment, including resistance to therapy, are not fully understood despite over 40 years of research. Here, we present X-ray structures of EcAII with an active site mutation, D90E, in three crystal forms. The region of the mutation is well ordered, allowing precise functional analysis of the consequences of the replacement of Asp90. In all three structures, the mutant protein exhibits an open conformation of the active site. In one of the structures, a zinc cation has been detected. The zinc cation is coordinated in a region of the protein that is implicated in the immunological response to EcAII treatment. A combined sequence-structure analysis of bacterial-type l-asparaginases reveals that the metal coordination may play a role in the response to asparaginase treatment. The observation of a zinc-binding site in antileukemic l-asparaginases provides new insight, with consequences for acute lymphoblastic leukemia therapy. The atomic coordinates of the monoclinic, orthorhombic and trigonal forms of the D90E mutant of Escherichia coli type II asparaginase have been deposited with the RCSB PDB with accession codes 1JAZ, 1JJA and 1IHD, respectively. EC 3.5.1.1, l-asparagine amidohydrolase, l-asparaginase; EC 3.5.1.38, l-glutamine (l-asparagine) amidohydrolase, glutaminase-asparaginase; EC 6.3.5.6, l-aspartyl-tRNA(Asn) : l-glutamine amido-ligase (ADP-forming), asparaginyl-tRNA synthase (glutamine-hydrolysing).
Organizational Affiliation:
Department of Crystallography, Faculty of Chemistry, A. Mickiewicz University, Poznan, Poland.