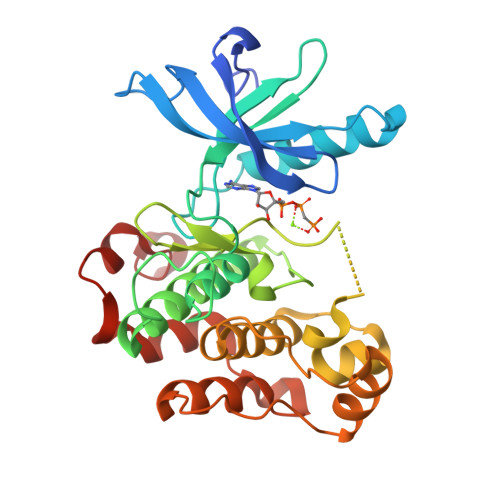
Crystallographic and solution studies of an activation loop mutant of the insulin receptor tyrosine kinase: insights into kinase mechanism.
Till, J.H., Ablooglu, A.J., Frankel, M., Bishop, S.M., Kohanski, R.A., Hubbard, S.R.(2001) J Biological Chem 276: 10049-10055
- PubMed: 11124964
- DOI: https://doi.org/10.1074/jbc.M010161200
- Primary Citation of Related Structures:
1I44 - PubMed Abstract:
The tyrosine kinase domain of the insulin receptor is subject to autoinhibition in the unphosphorylated basal state via steric interactions involving the activation loop. A mutation in the activation loop designed to relieve autoinhibition, Asp-1161 --> Ala, substantially increases the ability of the unphosphorylated kinase to bind ATP. The crystal structure of this mutant in complex with an ATP analog has been determined at 2.4-A resolution. The structure shows that the active site is unobstructed, but the end of the activation loop is disordered and therefore the binding site for peptide substrates is not fully formed. In addition, Phe-1151 of the protein kinase-conserved DFG motif, at the beginning of the activation loop, hinders closure of the catalytic cleft and proper positioning of alpha-helix C for catalysis. These results, together with viscometric kinetic measurements, suggest that peptide substrate binding induces a reconfiguration of the unphosphorylated activation loop prior to the catalytic step. The crystallographic and solution studies provide new insights into the mechanism by which the activation loop controls phosphoryl transfer as catalyzed by the insulin receptor.
Organizational Affiliation:
Skirball Institute of Biomolecular Medicine and Department of Pharmacology, New York University School of Medicine, New York, New York 10016, USA.