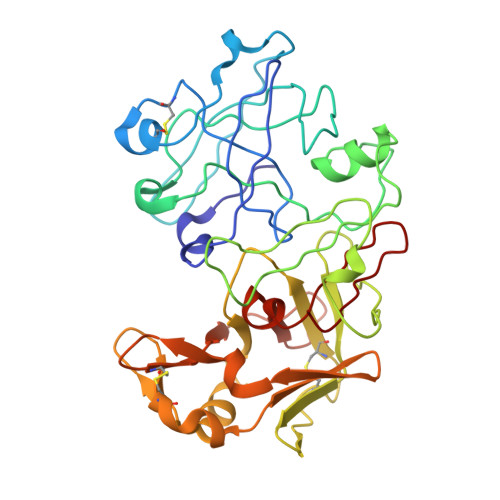
Crystal and molecular structures of human progastricsin at 1.62 A resolution.
Moore, S.A., Sielecki, A.R., Chernaia, M.M., Tarasova, N.I., James, M.N.(1995) J Mol Biol 247: 466-485
- PubMed: 7714902
- DOI: https://doi.org/10.1006/jmbi.1994.0154
- Primary Citation of Related Structures:
1HTR - PubMed Abstract:
The crystal and molecular structures of human progastricsin (hPGC) have been determined using multiple isomorphous replacement methods and anomalous scattering in conjunction with a phased translation function. The structure has been refined to a conventional R-factor (= sigma parallel Fo magnitude of - magnitude of Fc parallel / sigma magnitude of Fo magnitude of) of 0.179 with data to 1.62 A resolution. The first 37 amino acid residues of the prosegment are similar in conformation to the equivalent residues of porcine pepsinogen (pPGN). As in pPGN, the N zeta atom of Lys37p sits between the active-site carboxylate groups of Asp32 and Asp217, thereby preventing catalysis. The side-chains of Tyr38p and Tyr9 sit in the S1' and S1 substrate-binding pockets of hPGC, respectively, in an analogous manner to what is observed in porcine pepsinogen. There are large conformational differences centered around the region containing residues Arg39p to Pro6, relative to the equivalent region in the structure of pPGN. Two surface loops in the vicinity of this segment are also displaced relative to those in pPGN and in mature aspartic proteinases (Phe71 to Thr81 (the "flap"), and Tyr125 to Thr131). In hPGC, Tyr75 O eta does not make its usual hydrogen bond to Trp39 N epsilon 1. Rather, the "flap" containing Tyr75 is excluded from the active site by the polypeptide segment Arg39p to Pro6. However, the conformation of the inhibitory segment, Lys37p to Tyr38p, is virtually identical with that observed in pPGN. Hence the structures of these two proteins indicate that aspartic proteinase zymogens keep themselves inactive at neutral pH by a very similar mechanism in human progastricsin and porcine pepsinogen. This similarity likely carries over to all members of both the pepsinogen A and C families of aspartic proteinase zymogens.
Organizational Affiliation:
Department of Biochemistry, University of Alberta, Edmonton, Canada.